PD-L1, a Type I Interferon-regulated Immune Checkpoint Protein in Sepsis
Authors: Evan D. Shelton1, Yusen Liu1,2*
1Center for Perinatal Research, The Abigail Wexner Research Institute at Nationwide Children’s Hospital, Columbus, USA
2Department of Pediatrics, The Ohio State University College of Medicine, Columbus, OH 43205, USA
*Corresponding author: Professor. Yusen Liu, Center for Perinatal Research, The Abigail Wexner Research Institute at Nationwide Children’s Hospital, Columbus, OH 43215, USA; E-mail: yusen.liu@nationwidechildrens.org
Received: 13 July 2023; Revised: 02 August 2023; Accepted: 03 August 2023; Published: 08 August 2023
Copyright: © 2023 Shelton ED. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abbreviations: PD-L1: Programmed death-ligand 1; PD-1: Programmer death-1; EAE: Experimental autoimmune encephalomyelitis; E. coli: Escherichia coli; IFN: Interferon; IFNAR1: Interferon alpha and beta receptor 1; Mkp-1: Mitogen-activated protein kinase phosphatase 1; LPS: Lipopolysaccharide; JAK: Janus kinase; TYK2: Tyrosine kinase 2, WT: Wildtype
Running Title: Immunosuppression by PD-L1 Induced via IFN-I During Sepsis
Citation: Shelton ED, Liu Y (2023) PD-L1, a Type-I Interferon-regulated Immune Checkpoint Protein in Sepsis, 21st Century Pathology, Volume 3 (4): 150
Abstract
Programmed death-ligand 1 (PD-L1) is one of the best understood immune checkpoint proteins1,2. PD-L1 can inhibit the clonal expansion of CD8+ and CD4+ T lymphocytes through engaging with another inhibitory immune checkpoint molecule programmer death (PD)-1 expressed on T lymphocytes. In response to exogenous or endogenous danger signals, the adaptive immune system reacts to foreign antigens with rapid clonal expansion of antigen-specific CD8+ cytotoxic or CD4+ helper T lymphocytes. Engagement of PD-L1 with PD-1 leads to recruitment of tyrosine phosphatases SHP-2 via immunoreceptor tyrosine switch motif of PD-1, resulting in inhibition of the proliferation of antigen-specific T cells in lymph nodes and reduction of apoptosis of regulatory T cells3. It has been speculated that PD-L1 plays an important role in suppressing the adaptive immune system during pregnancy4, tissue allografts 5,6, and autoimmune disease7-9
Keywords:
Sepsis; Phosphatase; Immune checkpoint; Interferon; Inflammation
Description
Programmed death-ligand 1 (PD-L1) is one of the best understood immune checkpoint proteins [1,2]. PD-L1 expressed on antigen-presenting cells and tumor cells can inhibit the clonal expansion of CD8+ and CD4+ T lymphocytes through engaging with another inhibitory immune checkpoint molecule programmer death (PD)-1 expressed on T lymphocytes. In response to exogenous or endogenous danger signals, the adaptive immune system reacts to foreign antigens with rapid clonal expansion of antigen-specific CD8+ cytotoxic or CD4+ helper T lymphocytes. In T lymphocytes, engagement of PD-1 with PD-L1 leads to recruitment of tyrosine phosphatases SHP-2 via immunoreceptor tyrosine switch motif of PD-1, resulting in inhibition of the proliferation of antigen-specific T cells in lymph nodes or tumor environment and reduction of apoptosis of regulatory T cells [3]. It has been speculated that PD-L1 plays an important role in suppressing the adaptive immune system during pregnancy [4], tissue allografts [5,6], and autoimmune disease [7-9]. PD-1/PD-L1 interactions are important to prevent excessive immune-mediated tissue damage and autoimmunity [10]. It has been shown that PD-1-deficient mice are susceptible to develop lupus-like autoimmune disease [11,12], diabetes [13], or catastrophic autoimmune cardiomyopathy [14]. PD-L1 deficiency converted the 129S4/SvJae strain of mice from a resistant to experimental autoimmune encephalomyelitis (EAE)-susceptible strain [15]. A number of cancer cells express elevated PD-L1, making them resistant to killing by CD8+ T cells that recognize cancer-specific neoantigens. Administration of monoclonal antibodies against PD-L1 or PD-1 can unleash the T lymphocytes specific to the cancer-specific neoantigens, resulting in the killing of cancer cells [16]. Immune checkpoint blockade therapy targeting PD-1 and PD-L1 has revolutionized cancer treatment in the past decade [17]. Currently, a number of PD-1 and PD-L1 monoclonal antibodies have been approved by the United States Food and Drug Administration (FDA) for dozens of malignancies [1,18,19]. These PD-1/PD-L1 inhibitors significantly improved the overall survival of cancer patients and have emerged as the standard therapy for multiple malignancies [17,20].
Sepsis is associated with a cytokine storm, frequently followed by shock and multi-organ system failure [21]. As sepsis progresses, patients often develop immunoparalysis associated with massive splenic lymphocyte apoptosis [22]. The majority of shock-related deaths occur during this hypo-immune state, likely due to a failure in clearing primary or secondary (nosocomial) infections [23,24]. In recent years, numerous studies provided ample evidence to support a role of PD1/PD-L1 in sepsis. In a prospective cohort study Shao R, et al. (2016) found that increased monocyte PD-L1 expression following sepsis is associated with risk stratification and mortality in septic patients [25,26]. Guignant C, et al. (2011) found that increased PD 1 levels are associated with increased mortality, nosocomial infection, and immune dysfunction in septic shock patients [27]. Wang JF, et al. (2015) proposed that increased PD-L1 expression on neutrophils could be involved in immunoparalysis [28] that is often seen in sepsis patients [22,24]. Zhang Y, et al. (2011) showed that PD-L1 expression was elevated in blood monocytes of sepsis patients [26]. Blockade of PD-L1 on blood monocytes from sepsis patients enhanced the production of TNF-α and IL-6 after LPS stimulation [26]. Patera AC, et al. (2016) demonstrated that incubation of neutrophils and monocytes of sepsis patient with a monoclonal PD-L1 antibody in vitro enhanced the phagocytosis activity against E. coli [29]. In murine models of sepsis, administration of ant-PD-L1 antibodies or peptide has been shown to diminish lymphocyte apoptosis, reduce bacterial/fungal burden, and decrease mortality [30-32]. Chang KC, et al. (2013) studied the effects of immune checkpoint blockade on survival and immunosuppression using PD-L1 and PD-1 antibodies in animal models of primary Candidemia or secondary fungemia following sublethal cecal ligation and puncture [33]. They demonstrated that anti-PD-L1 and PD-1 antibodies, when administered 24 to 48 h after fungal infection, were highly effective at improving animal survival in both primary and secondary fungal sepsis. Both anti-PD-1 and anti-PD-L1 antibodies reversed sepsis-induced suppression of IFN-γ and increased MHC II expression on antigen presenting cells. Burn patients are particularly susceptible to infections due, in part, to immune dysfunction. Patil NK, et al. (2018) studied the effect of PD-L1 blockade on immune dysfunction after burn injury, using a mouse model of burn injury and bacterial sepsis [34]. They found that burn injury and subsequent infection with Pseudomonas aeruginosa caused a significant upregulation of PD-L1 on myeloid cells, along with a decrease in T cell numbers and function, significant multiorgan injury, and decreased survival. After burn injury, treatment with an anti-PD-L1 antibody improved bacterial clearance, reduced organ injury, and enhanced survival after infection by both P. aeruginosa and Staphylococcus aureus. Taken together, these studies suggest that blocking PD-1/PD-L1 axis could prevent the sepsis-associated immunoparalysis and improve patient outcomes.
The paper titled "Enhanced PD-L1 expression contributes to the bactericidal defect of Mkp-1-deficient mice during Escherichia coli infection" explores the impact of mitogen-activated protein kinase phosphatase 1 (Mkp-1) deficiency on immune defense during systemic Escherichia coli infection. In addition to investigating the role of Mkp-1, the study sheds light on the potential interplay between PD-L1, inflammation, type I interferon, and bacterial pathogen E. coli. They found that PD-L1 was induced prominently in macrophages in the spleen and livers after E. coli infection, and the induction in these cells was markedly stronger in Mkp-1 knockout mice than in wildtype (WT) mice. In an earlier study, the same group found that Mkp-1 knockout mice exhibited an enhanced cytokine production after systemic E. coli infection, associated with elevated mortality, severe metabolic abnormality, and defective bactericidal activity [35]. Despite the enhanced inflammatory response, bacterial burdens were significantly higher in the Mkp-1 knockout mice than in the WT mice [35]. In another earlier study, this group found that large group of cytokines, including TNF-α, IL-1α/β, IL-6, IL-10, IL-17A, IL-27, GM-CSF, and type I interferon IFN-β were more robustly produced in Mkp-1 knockout mice than in WT mice [36]. To understand the role of Mkp-1 in the regulation of global gene expression, they performed RNA-seq analysis on liver tissues collected from WT and Mkp-1 knockout mice both before and after E. coli infection [37]. They found that the expression of 5,369 and 7,251 genes were altered in WT and Mkp-1 knockout mice, respectively, after systemic E. coli infection, and 5421 genes were differentially expressed between WT and Mkp-1 knockout mice after E. coli infection. Supporting the notion that elevated type I interferon has a considerable impact on host defense, they found that 60 of the 71 known interferon-inducible genes were upregulated after systemic E. coli infection in WT mice, and 40 of the 71 known interferon-inducible genes were more robustly induced after E. coli infection in Mkp-1 knockout mice than in WT mice [36]. Blockade of the receptor of type I interferon, IFNAR1, with a monoclonal antibody almost totally suppressed the induction of Rsad2, a classical type I interferon-induced gene, but augmented mortality and disease severity, suggesting that type I interferon is beneficial in E. coli-infected Mkp-1 knockout mice. In the studies published in the Journal Biological Chemistry, Barley TJ, et al. (2022) found that IFNAR1 blockade with a monoclonal antibody almost totally abolished PD-L1 expression in the livers of E. coli-infected Mkp-1 knockout mice, indicating a central role of type I interferon in PD-L1 expression [38]. To address the role of PD-L1 in Mkp-1 knockout mice during systemic E. coli infection, the group blocked PD-L1 with a monoclonal antibody and assessed the effect of PD-L1 blockade on animal survival and bacterial burdens [38]. Interestingly, although PD-L1 blockade in E. coli-infected Mkp-1 knockout mice decreased bacterial burdens, the mortality of the mice administered with the PD-L1-blocking monoclonal antibody was actually increased relative to mice that received the isotype control antibody. These results suggest that PD-L1 expression in Mkp-1 knockout mice is actually beneficial to the mice. Given the fact that blockade of type I interferon signaling also increased the mortality of the E. coli-infected Mkp-1 knockout mice [36], these results strongly suggest that the beneficial effect of type I interferons is mediated primarily through PD-L1. To understand the mechanism for the increased mortality in Mkp-1 knockout mice, Barley TJ, et al. (2022) examined the effect of PD-L1 blockade on inflammatory indices. They found that PD-L1 blockade significantly enhanced the TNF-α and IFN-γ levels in the serum as well as iNOS levels in the lung and liver tissues, suggesting that PD-L1 can inhibit the propagation of the inflammatory response. To understand the regulation of PD-L1, the authors examined PD-L1 induction in bone marrow-derived macrophages stimulated with E. coli or lipopolysaccharide (LPS), a major component of the cell wall of Gram-negative bacteria. They showed that PD-L1 was potently induced in macrophages by both E. coli and LPS in vitro, and Mkp-1 deficiency exacerbated PD-L1 induction with little effect on the half-life of PD-L1 mRNA. In contrast, inhibitors of Janus kinase (JAK) 1/2 and Tyrosine kinase (TYK) 2, as well as the IFNAR1-neutralizing monoclonal antibody, markedly attenuated PD-L1 induction in macrophages. These studies established that Mkp-1 controls the production of type I interferons, which drive PD-L1 expression via the JAK/STAT pathway in an autocrine manner (Fig. 1). Through this mechanism, during sepsis Mkp-1 indirectly regulates PD-L1 expression, and executes its feedback control over the downstream inflammatory process, likely through T-cell-mediated mechanisms.
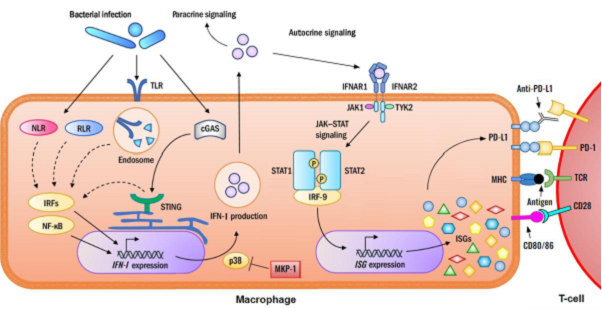
Figure 1: Immunosuppression by PD-L1 induced through type I interferon during bacterial sepsis. During bacterial infection, microbial components interact with pathogen pattern recognition receptors (TLR, NLR, RLR, and cGAS), in macrophages leading to the activation of transcription factors IRF and NF-κB as well as the p38 MAPK pathways. Both IRF and NF-κB positively regulate the expression of type I interferon (IFN-I) gene. The p38 MAPK pathway stabilizes the mRNAs of IFN-I and enhances IFN-I protein translation. IFN-I then regulates host defense through both autocrine and paracrine (not shown in the diagram) mechanisms. In the autocrine mechanism, IFN-I binds to the IFN-I receptor on the cell surface and activates the JAK/STAT pathway to induce the IFN-stimulated genes (ISGs), including PD-L1. PD-L1 dimerizes with its receptor PD-1 on T cells, contributing to immunosuppression. An PD-L1 antibody binds to PD-L1 and breaks the interaction with PD-1, thus alleviating PD-L1-mediated immunosuppression of T lymphocytes. As a feedback control regulator of MAPK pathways, Mkp-1 switches off p38 and limits the production of IFN-I and PD-L1, thereby maintaining immunocompetence.
The decreased survival in Mkp-1 knockout mice after PD-L1 blockade contradicts the improved survival after PD-L1 blockade in previous animal models of bacteremia or fungemia. The discrepancy could potentially be attributed to the varying degrees of inflammation in different infection models. In the present study, E. coli were introduced into the blood circulation of Mkp-1 knockout mice. It is well known that Mkp-1 deficiency dramatically enhances the production of a variety of inflammatory and anti-inflammatory cytokines. In fact, Mkp-1 knockout mice produced 7 and 9 times more TNF-α and IL-6, respectively, than the WT mice 24 h after E. coli infection [35]. PD-L1 blockade further enhanced the production of at least TNF-α, IFN-γ, and iNOS, further exacerbating the hyper-inflammatory response of Mkp-1 knockout mice. It is likely that the overly exaggerated inflammatory response leads to further enhanced iNOS induction, devastating vasodilation, and a dramatic decrease in blood pressure, which result in further increased mortality. In this sense, the beneficial effect of PD-L1 blockade on bacteria-killing had no effect on overall survival of the animals. In hindsight, this is not surprising, since previously it has been shown that complete killing of bacteria by gentamicin three hours after E. coli infection did not prevent the death caused by E. coli infection, although mortality in WT mice was completely prevented. In the studies where PD-L1 blockade resulted in a reduction in mortality and organ damage [30-32,34], wildtype mice were used. Therefore, it is reasonable to speculate that the inflammatory responses in these situations were less robust than in Mkp-1 knockout mice. We speculate that under such circumstances, inhibiting PD-L1 might be beneficial as it enhances immune-mediated clearance of the pathogen and prevents immune exhaustion. With improved pathogen clearance, pathogen-cause inflammatory response also subdued. In these contexts, blocking PD-L1 can enhance the pathogen-killing activity of the phagocytotic cells through restoring the activity of pathogen-specific T cells and other effector immune cells, leading to improved survival outcomes. It is tempting to speculate that PD-L1 blockade is likely more beneficial to sepsis cases with depressed inflammatory response, such as in patients with immunoparalysis or with a less inflammatory pathogens, such as Candida auris. A phase 1b randomized study evaluating the safety, tolerability, pharmacokinetics, and pharmacodynamics of the PD-1 inhibitor nivolumab and the PD-L1 inhibitor BMS-936559 in sepsis patients has been completed [39,40].
Conclusion
The studies indicate that PD-L1 blockade was safe for the treatment of sepsis, although a larger clinical trial is needed to assess the efficacy of immune checkpoint inhibitor therapy for the treatment of sepsis. With clinical trials in progress, there is promise that regulation of PD-L1 will provide optimism for those with or at risk of developing sepsis. Recent advances in the understanding of the PD-L1/PD-1 axis have also unlocked the potential for a therapeutic target for sepsis-associated immunoparalysis. In addition, the recent finding that IFNAR1 acts as a positive regulator of PD-L1 in sepsis raises an interesting question of whether the FDA-approval type I interferon receptor antagonist for systemic lupus erythematosus, SAPHNELO, may also show efficacy in treating bacterial or fungal sepsis.
Acknowledgement
This work was supported by grants from NIH (AI124029 and AI142885).
References
1. Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018 Mar 20;48(3):434-52. https://doi.org/10.1016/j.immuni.2018.03.014
2. Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027-34. https://doi.org/10.1084/jem.192.7.1027
3. Patsoukis N, Duke-Cohan JS, Chaudhri A, Aksoylar HI, Wang Q, Council A, et al. Interaction of SHP-2 SH2 domains with PD-1 ITSM induces PD-1 dimerization and SHP-2 activation. Commun Biol. 2020;3(1):128. https://doi.org/10.1038/s42003-020-0845-0
4. Zhang YH, Tian M, Tang MX, Liu ZZ, Liao AH. Recent Insight into the Role of the PD-1/PD-L1 Pathway in Feto-Maternal Tolerance and Pregnancy. Am J Reprod Immunol. 2015;74(3):201-8. https://doi.org/10.1111/aji.12365
5. McGrath MM, Najafian N. The role of coinhibitory signaling pathways in transplantation and tolerance. Front Immunol. 2012;3:47. https://doi.org/10.3389/fimmu.2012.00047
6. Riella LV, Paterson AM, Sharpe AH, Chandraker A. Role of the PD-1 pathway in the immune response. Am J Transplant. 2012;12(10):2575-87. https://doi.org/10.1111/j.1600-6143.2012.04224.x
7. Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219-42. https://doi.org/10.1111/j.1600-065X.2010.00923.x
8. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6(1):38. https://doi.org/10.1038/s41572-020-0160-6
9. Burke KP, Patterson DG, Liang D, Sharpe AH. Immune checkpoint receptors in autoimmunity. Curr Opin Immunol. 2023;80:102283. https://doi.org/10.1016/j.coi.2023.102283
10. Merelli B, Massi D, Cattaneo L, Mandalà M. Targeting the PD1/PD-L1 axis in melanoma: biological rationale, clinical challenges and opportunities. Crit Rev Oncol Hematol. 2014;89(1):140-65. https://doi.org/10.1016/j.critrevonc.2013.08.002
11. Chua KH, Lian LH, Sim XJ, Cheah TE, Lau TP. Association between PDCD1 Gene Polymorphisms and Risk of Systemic Lupus Erythematosus in Three Main Ethnic Groups of the Malaysian Population. Int J Mol Sci. 2015;16(5):9794-803. https://doi.org/10.3390/ijms16059794
12. Nishimura H, Honjo T, Minato N. Facilitation of beta selection and modification of positive selection in the thymus of PD-1-deficient mice. J Exp Med. 2000;191(5):891-8. https://doi.org/10.1084/jem.191.5.891
13. Godoy GJ, Olivera C, Paira DA, Salazar FC, Ana Y, Stempin CC, et al. T Regulatory Cells From Non-obese Diabetic Mice Show Low Responsiveness to IL-2 Stimulation and Exhibit Differential Expression of Anergy-Related and Ubiquitination Factors. Front Immunol. 2019;10:2665. https://doi.org/10.3389/fimmu.2019.02665
14. Wang J, Okazaki IM, Yoshida T, Chikuma S, Kato Y, Nakaki F, et al. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol. 2010;22(6):443-52. https://doi.org/10.1093/intimm/dxq026
15. Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A. 2004;101(29):10691-6. https://doi.org/10.1073/pnas.0307252101
16. Burr ML, Sparbier CE, Chan YC, Williamson JC, Woods K, Beavis PA, et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature. 2017;549(7670):101-5. https://doi.org/10.1038/nature23643
17. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350-5. https://doi.org/10.1126/science.aar4060
18. Sanmamed MF, Chen L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell. 2018;175(2):313-26. https://doi.org/10.1016/j.cell.2018.09.035
19. Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol. 2017;8:561. https://doi.org/10.3389/fphar.2017.00561
20. Shen X, Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. Bmj. 2018;362:k3529. https://doi.org/10.1136/bmj.k3529
21. Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420(6917):885-91. https://doi.org/10.1038/nature01326
22. Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13(3):260-8. https://doi.org/10.1016/S1473-3099(13)70001-X
23. Cornell TT, Sun L, Hall MW, Gurney JG, Ashbrook MJ, Ohye RG, et al. Clinical implications and molecular mechanisms of immunoparalysis after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2012;143(5):1160-6. https://doi.org/10.1016/j.jtcvs.2011.09.011
24. Frazier WJ, Hall MW. Immunoparalysis and adverse outcomes from critical illness. Pediatr Clin North Am. 2008;55(3):647-68. https://doi.org/10.1016/j.pcl.2008.02.009
25. Shao R, Fang Y, Yu H, Zhao L, Jiang Z, Li CS. Monocyte programmed death ligand-1 expression after 3-4 days of sepsis is associated with risk stratification and mortality in septic patients: a prospective cohort study. Crit Care. 2016;20(1):124-1301. https://doi.org/10.1186/s13054-016-1301-x
26. Zhang Y, Li J, Lou J, Zhou Y, Bo L, Zhu J, et al. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit Care. 2011;15(1):R70. https://doi.org/10.1186/cc10059
27. Guignant C, Lepape A, Huang X, Kherouf H, Denis L, Poitevin F, et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care. 2011;15(2):R99. https://doi.org/10.1186/cc10112
28. Wang JF, Li JB, Zhao YJ, Yi WJ, Bian JJ, Wan XJ, et al. Up-regulation of programmed cell death 1 ligand 1 on neutrophils may be involved in sepsis-induced immunosuppression: an animal study and a prospective case-control study. Anesthesiology. 2015;122(4):852-63. https://doi.org/10.1097/ALN.0000000000000525
29. Patera AC, Drewry AM, Chang K, Beiter ER, Osborne D, Hotchkiss RS. Frontline Science: Defects in immune function in patients with sepsis are associated with PD-1 or PD-L1 expression and can be restored by antibodies targeting PD-1 or PD-L1. J Leukoc Biol. 2016;100(6):1239-54. https://doi.org/10.1189/jlb.4HI0616-255R
30. Zhang Y, Zhou Y, Lou J, Li J, Bo L, Zhu K, et al. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit Care. 2010;14(6):R220. https://doi.org/10.1186/cc9354
31. Zhu W, Bao R, Fan X, Tao T, Zhu J, Wang J, et al. PD-L1 blockade attenuated sepsis-induced liver injury in a mouse cecal ligation and puncture model. Mediators Inflamm. 2013;2013:361501. https://doi.org/10.1155/2013/361501
32. Shindo Y, McDonough JS, Chang KC, Ramachandra M, Sasikumar PG, Hotchkiss RS. Anti-PD-L1 peptide improves survival in sepsis. J Surg Res. 2017;208:33-39. https://doi.org/10.1016/j.jss.2016.08.099
33. Chang KC, Burnham CA, Compton SM, Rasche DP, Mazuski RJ, McDonough JS, et al. Blockade of the negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Crit Care. 2013;17(3):R85. https://doi.org/10.1186/cc12711
34. Patil NK, Luan L, Bohannon JK, Hernandez A, Guo Y, Sherwood ER. Frontline Science: Anti-PD-L1 protects against infection with common bacterial pathogens after burn injury. J Leukoc Biol. 2018;103(1):23-33. https://doi.org/10.1002/JLB.5HI0917-360R
35. Frazier WJ, Wang X, Wancket LM, Li XA, Meng X, Nelin LD, et al. Increased inflammation, impaired bacterial clearance, and metabolic disruption after gram-negative sepsis in Mkp-1-deficient mice. J Immunol. 2009;183(11):7411-9. https://doi.org/10.4049/jimmunol.0804343
36. Kirk SG, Murphy PR, Wang X, Cash CJ, Barley TJ, Bowman BA, et al. Knockout of MAPK Phosphatase-1 Exaggerates Type I IFN Response during Systemic Escherichia coli Infection. J Immunol. 2021;206(12):2966-79. https://doi.org/10.4049/jimmunol.2001468
37. Li J, Wang X, Ackerman WEt, Batty AJ, Kirk SG, White WM, et al. Dysregulation of Lipid Metabolism in Mkp-1 Deficient Mice during Gram-Negative Sepsis. Int J Mol Sci. 2018;19(12):3904. https://doi.org/10.3390/ijms19123904
38. Barley TJ, Murphy PR, Wang X, Bowman BA, Mormol JM, Mager CE, et al. Mitogen-Activated Protein Kinase Phosphatase-1 Controls PD-L1 Expression by Regulating Type I Interferon during Systemic Escherichia coli Infection. J Biol Chem. 2022:101938. https://doi.org/10.1016/j.jbc.2022.101938
39. Hotchkiss RS, Colston E, Yende S, Crouser ED, Martin GS, Albertson T, et al. Immune checkpoint inhibition in sepsis: a Phase 1b randomized study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of nivolumab. Intensive Care Med. 2019;45(10):1360-71. https://doi.org/10.1007/s00134-019-05704-z
40. Hotchkiss RS, Colston E, Yende S, Angus DC, Moldawer LL, Crouser ED, et al. Immune Checkpoint Inhibition in Sepsis: A Phase 1b Randomized, Placebo-Controlled, Single Ascending Dose Study of Antiprogrammed Cell Death-Ligand 1 Antibody (BMS-936559). Crit Care Med. 2019;47(5):632-42. https://doi.org/10.1097/ccm.0000000000003685
