Short Commentary - Giant Magnetoresistance-Based Biosensors for Next Generation High throughput, Point-of-Care Cancer Diagnosis
Authors: Shuang Liang1, Kai Wu2 and Jian-Ping Wang1,2*
1Department of Chemical Engineering and Materials Science, University of Minnesota, Minneapolis, MN 55455, USA
2Department of Electrical and Computer Engineering, University of Minnesota, Minneapolis, MN 55455, USA
*Correspondence to: : Jian-Ping Wang, PhD, Distinguished McKnight University, Professor & Robert F Hartmann Chair, Professor, Department of Electrical and Computer Engineering, University of Minnesota, USA; E-mail: jpwang@umn.edu
Received: 25 March 2022; Revised: 03 April 2022; Accepted: 08 April 2022; Published: 14 April 2022
Copyright:
Citation: Shuang Liang, Kai Wu and Jian-Ping Wang (2022) Short Commentary - Giant Magnetoresistance-Based Biosensors for Next Generation High throughput, Point-of-Care Cancer Diagnosis, 21st Century Pathology, Volume 2 (2): 118
Abstract
The last decade has seen the flourishing development of giant magnetoresistance (GMR)-based sensors in the areas of hard-disk drives, bioassays, magnetic field sensors, and microelectromechanical systems (MEMS). Benefiting from the state-of-the-art thin film deposition and nanofabrication techniques, GMR sensor arrays are actively used in large-scale, high-throughput disease biomarkers screening, and brain and cardiac mapping. Herein, we briefly introduce the concept of spintronics and spintronic devices. Specifically, the physics of the GMR effect and the three different types of GMR structures are discussed. Methods for GMR sensor surface functionalization and GMR-based bioassay mechanisms are presented. Several GMR-based point-of-care (POC) platforms are reviewed. The opportunities and challenges in providing one-step, wash-free, low-cost, rapid, and accurate GMR POC platforms for future high throughput disease screening is commented on at the end of this commentary.
Keywords:
Giant magnetoresistance; Spintronics; Cancer diagnosis; Point-of-care; Biosensors
Spintronics
Spintronics is one of the most rapidly emerging research areas in these years [1-3]. While traditional electronic devices are manipulating the charge of the electron, spintronic devices are also exploiting the spin of the electron. To date, the most often studied spintronic devices are anisotropic magnetoresistance (AMR) device, giant magnetoresistance (GMR) device, magnetic tunnelling junction (MTJ), and spin-torque oscillator (STO), etc. Many of them have been used practically in magnetic random access memory (MRAM) and hard disk drive (HDD) read head [4-6]. Besides tremendous applications in data storage and information transport, spintronic devices also show great potential in biomedical areas. For example, GMR and MTJ magnetic field sensors have been widely used in disease diagnosis and biological activity recording (e.g., brain and cardiac imaging) [7-11]. Specifically, a biosensing platform based on GMR is a robust method for quantitatively detecting biological analytes such as proteins, nucleic acid, and whole cells. It has been proved by many research groups that GMR biosensors can detect foodborne pathogens, toxins, cancer biomarkers, etc., with high sensitivity [12-14]. Compared to conventional bioassay platforms such as enzyme-linked immunosorbent assay (ELISA), GMR biosensors use magnetic labels instead of fluorescence tags for quantitative detection of target biomarkers. The biological samples usually are nonmagnetic, which enables a lower background noise level, and thus a better detection limit of GMR biosensors than optical biosensors. Also, unlike optical labels, magnetic labels are more stable and will not lose their integrity over time [15]. All of these benefits make GMR biosensors competitive candidates for sensing platforms in food safety and disease diagnosis.
Giant Magnetoresistance (GMR) Effect
GMR effect is the giant electrical resistance change of metallic layered structures when the magnetizations in the ferromagnetic (FM) layers are reoriented upon the application of an external magnetic field. This effect was firstly reported in 1988 by the Albert Fert and Peter Grunberg teams, independently [16, 17] Later recognized by the 2007 Nobel Prize in Physics. GMR is a quantum mechanics phenomenon found in multilayers composed of alternating ferromagnetic (FM) and nonmagnetic (NM) conductive layers. The physical origin of the GMR effect is the spin-dependent scattering of the conducting electrons. In a magnetically ordered material, the electrical resistance of the system is dependent on the scattering of conducting electrons on the magnetic sub lattices of the crystals. This scattering is weaker when the electron spin and magnetization in the FM layer are parallel and stronger when antiparallel. For a more detailed explanation of spin-dependent scattering as well as the GMR working principal, please refer to Reference [8].
Different Types of GMR Sensors
As is aforementioned, the GMR effect is reported in magnetic multilayer systems consisting of alternating ferromagnetic (FM) and nonmagnetic (NM) conductive layers, as shown in Figure 1(A). The electrical resistance of a GMR FM/NM multilayer system is relatively low when the magnetizations of neighboring FM layers are in parallel alignment (denoted as Rap), whereas the resistance is relatively high when in antiparallel alignment (denoted as Rap). One important characteristic of GMR structure is the GMR ratio, which is defined as (Rap-Rp)/Rp, unit: %. This GMR ratio and the detectivity are two figures of merit to evaluate the performance of GMR sensors. The detectivity is also known as the field equivalent noise level, which is corresponding to the noise spectra divided by the sensitivity [18]. A detailed definition of detectivity can be found in Reference [15].
The second type of GMR structure is the spin valve. As shown in Figure 1(B), spin valves are tri-layer structures that consist of two FM layers separated by a thin NM conducting layer. The antiferromagnetic (AFM) layer underneath is serving as the pinning layer to fix the magnetization in the neighboring FM layer to one direction (also called the “pinned layer”). While the magnetization of the other FM layer is free to rotate, also called the “free layer”. The spin valves are more widely used in biomedical applications due to their linear R-H transfer curve and simple layer structure. This linear response curve allows for quantitative detection of bio-magnetic fields or magnetically labeled biomarkers for disease diagnosis.
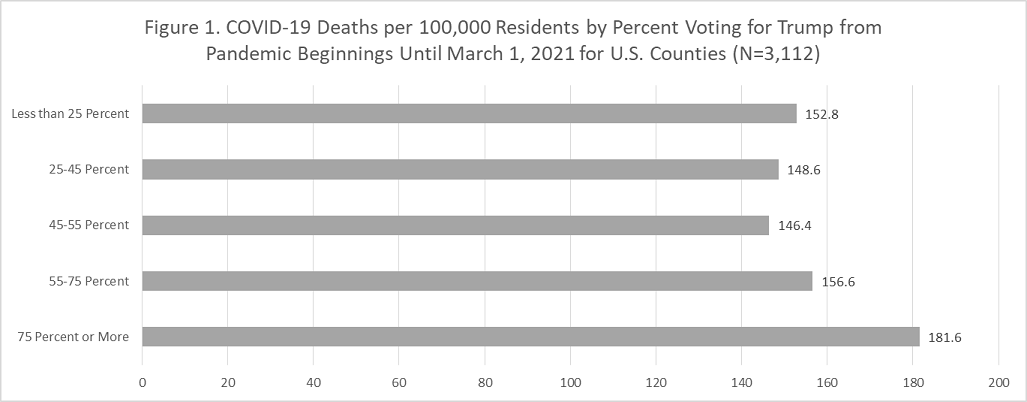
Figure 1: Schematic views of (A) GMR multilayer structure, (B) GMR spin valve structure, and (C) granular GMR, and the R-H transfer curves of each type of GMR structure.
The third type of GMR structure is named granular GMR, denoted as g-GMR, as shown in Figure 1(C). The g-GMR is firstly reported by Berkowitz AE, et al. (1992) and Xiao G, et al. (1993) [19, 20]. In this kind of GMR system, magnetic nanoparticles (MNPs) are either dispersed in conductive gel matrices or embedded in metallic matrices.
GMR Biosensors: Surface Functionalization and Bioassay Strategies
For GMR-based bioassays, the GMR sensor surfaces are typically deposited with insulating layers such as SiO2, Al2O3, Si3N4, etc. [21, 22]. This insulating layer has three major functions: 1) Isolate the GMR sensors from the external biological environment since some bio fluidic samples are corrosive and some magnetic materials from GMR thin film stacks are cytotoxic; 2) Prevent the leakage current from GMR sensors to the biological samples; 3) Lay the foundation for next step GMR sensor surface chemical modification. This thin insulating layer should be biocompatible and non-toxic; thus, the chemical vapor deposition (CVD) technique is not allowed for depositing this layer, and physical vapor deposition (PVD) is suggested by Moretti D, et al. (2018) [23]. In addition since the magnetic signal from the magnetic label decays fast over distance, this insulating layer is recommended to be thinner to avoid degrading the GMR sensor sensitivity and thick enough to effectively isolate the sensors from the external environment. Therefore, the insulating layer is usually reported in the range of tens to hundreds of nanometres thick. On top of this insulating layer, the GMR sensor surface is further chemically modified for bioassay purposes. To achieve the best effect of surface chemical modification, the GMR sensor chips should be cleaned thoroughly to remove contaminants. For example, the GMR chips are sequentially washed with acetone, methanol, isopropanol, and de-ionized water, blow-dried, then exposed to oxygen plasma or UV ozone for several minutes to remove organic residues. There are two most popular methods for sensor surface modification: 1) The APTES and Glu method [24, 25]; 2) The NHS and EDC method [21, 26]. Both modification methods activate the GMR sensor surface for effective coupling with free amine containing biomolecules such as the capture antibodies, oligonucleotides and aptamers.
GMR Biosensor Arrays for Multiplexed Cancer Biomarker Detection
GMR biosensors use magnetic labels, such as magnetic nanoparticles (MNPs) and magnetic beads (MBs), for quantitative and specific detection of target biomarkers. For simplicity, these magnetic labels are collectively referred to as MNPs in this paper. The MNPs bind to target biomarkers through specific antibody-antigen or DNA/DNA recognition and are immobilized on the GMR sensor surface (see Figures 2(A) & (B)). Under an external magnetic field, the immobilized MNPs generate magnetic stray fields that changes the electrical resistance (as well as MR ratio) of the GMR sensor, as shown in Figures 2(C) & (D). This resistance change, ΔR, or MR ratio change, ΔMR, is proportional to the number of MNPs captured to the sensor surface, which in turn proportional to the number of targeted biomarkers. Quantitative detection of the biomarkers can then be realized. Depending on the type of the biomarkers, many different structures of bioassays have been adapted [27-30]. If cancer biomarkers are DNA fragments, then the assays are usually DNA-based assays with a typical structure shown in Figure 2(A). If biomarkers are cancer cells or antigens, the assays are usually built based on specific binding between antibodies and antigens. The most common bioassays, in this case, are the sandwich assay, of which the structure is shown in Figure 2(B) [31]. The establishment of such bioassay starts with immobilizing capture antibodies onto the GMR sensor surface. Next, targeted antigens specifically bind to capture antibodies, and then the biotinylated detection antibodies are added and specifically bind to antigens. Finally, streptavidin-coated MNPs bind to detection antibodies via the interaction of streptavidin and biotin. The whole building-up process can take over ten hours due to the involvement of multiple incubation and washing steps.
Benefiting from powerful nanofabrication techniques, tens to hundreds or thousands of GMR sensors can be integrated with an area the size of a fingernail. For example, Hall D, et al. (2013) reported a 256-pixel GMR biosensor array in 0.18 μm CMOS. They demonstrated a real-time detection of an ovarian cancer biomarker, secretory leukocyte peptidase inhibitor (SLPI), with a limit of detection (LOD) of 10 fM [32]. Assembled in one chip, GMR sensors work independently, and, by functionalizing different capture probes on individual GMR sensors, it is possible to detect more than one type of target biomarkers from one sample, in one test. This kind of multiplexed bioassay not only improves the accuracy of diagnosing particular cancer but also increases the efficiency of diagnosing by screening for various cancers simultaneously (see Figure 2(E)). For example, Klein et al. successfully quantified three different ovarian cancer biomarkers on a single 4 × 4 GMR sensor array. To realize multiplex detection, three types of capture antibodies are used. Those are antibodies against cancer antigen 125 (CA125 II), human epididymis protein 4 (HE4), and interleukin 6 (IL6). During the detection process, those three capture antibodies and negative control of bovine serum albumin (BSA) were printed on individual sensors in four replicates [27]. The negative control of BSA was used as a reference for the background signal level. By establishing such a multiplex assay, multiplex detection of CA125 II, HE4 and IL6 were successfully achieved with LOD of 3.7 U/mL, 7.4 pg/mL, and 7.4 pg/mL, respectively. The real-time testing results of these ovarian biomarkers are shown in Figures 3(C) & (D). Sharing the same idea of using multiple biomarkers, Gao Y, et al. (2019) reported a multiplexed detection of twelve tumor biomarkers on one GMR chip containing 40 individual sensors [30]. They functionalized individual GMR sensors with different capture antibodies. Another example of multiplex detection of cancer biomarkers was reported by Xu L, et al. (2019) for prostate cancer (CaP) diagnosis [33]. They developed a multiplex assay to detect six biomarkers on a single chip, including four CaP-related autoantibodies, prostate-specific antigen (PSA), and free/total PSA ratio. In addition to cancer diagnostics, multiplexed GMR biosensors are also capable of monitoring response to cancer therapy. For instance, Nesvet J, et al. (2021) analyzed different circulating tumor DNA (ctDNA) epidermal growth factor receptor (EGFR) mutations with a GMR sensors array [34]. This analysis is critical for selecting appropriate treatment options for patients with non-small cell lung cancer (NSCLC). Combined with PCR amplification, they reached a detection sensitivity of 87.5% and 90%, for Exon19 deletion and L858R mutation, respectively.
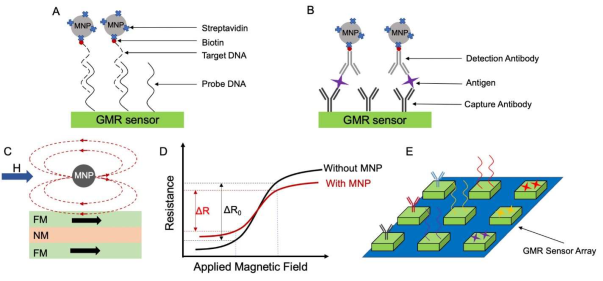
Figure 2: Schematic diagrams of (A) DNA-based assay structure and (B) Sandwich assay structure. (C) MNPs generate stray fields that alter the magnetization in the underlying FM layer, thus, causes the resistance and MR ratio change in (D). (E) A multiplexed bioassay on a GMR sensor array.
Conventional detection of cancer biomarkers usually occurs in laboratories and requires well-trained staff to perform the test, which is often expensive and time-consuming. Therefore, point-of-care (POC) devices have attracted increasing research interest since they are portable, easy to operate, and cost-efficient. The small size of the GMR chip allows it to be integrated into portable devices, making the GMR biosensing platform a good candidate for POC devices. To date, GMR-based POC diagnostic platforms have been reported by several research groups. One example of GMR POC devices is the Z-Lab developed by the University of Minnesota research group, as shown in Figures 3(A) & (B) [24]. The Z-Lab platform consists of a disposable cartridge used for sample loading, a handheld device acting as the reader station, and an electrical interface connecting the GMR chip to the device (see Figure 3(B)). Z-Lab can communicate with smartphones, tablets, and computers wirelessly or via a USB connection. The research group from Stanford University also reported a similar GMR POC device, the Eigen Diagnosis Platform [35] This platform has been demonstrated to be able to detect HIV and leukocytosis. Another example of a GMR-based POC platform was reported by one group from the Chinese Academy of Sciences [30]. Compared to the other two platforms, this one has an additional micro fluid channel system, which makes a fully automatic bioassay achievable. Therefore, a future direction for GMR POC devices would be integrating microfluidic channels into the sensing platform. Such microfluidic channels are often made by injecting polymeric materials such as PDMS into molds [36, 37] Challenges of the integration lie in the design of the channel and the bonding between the channel and the GMR chip. The development of GMR POC devices can be largely promoted if the cost of the individual fully packed microfluidic package can be resolved.
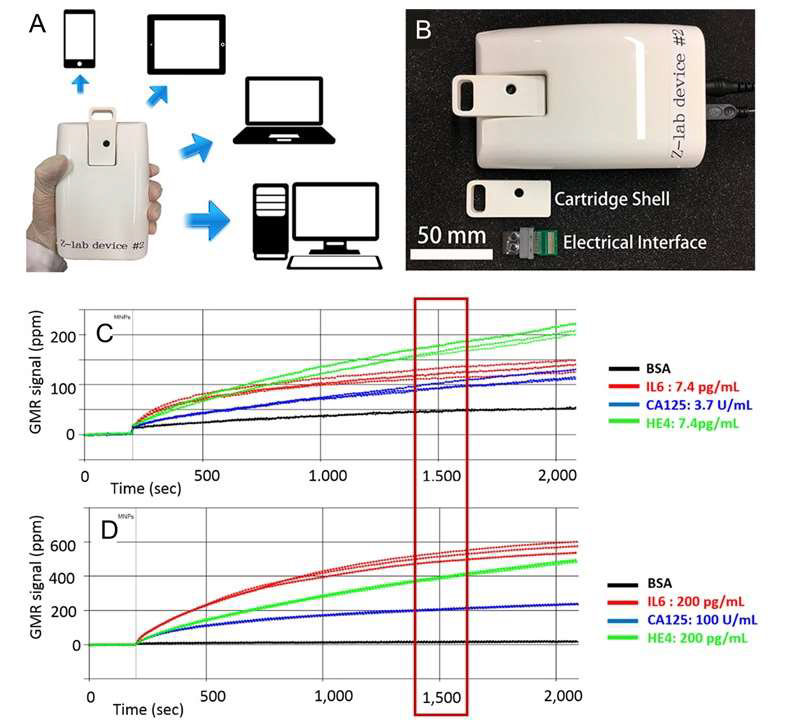
Figure 3: (A) Z-Lab device can communicate with smartphones, tablets, laptops, and desktop computers. (B) An optical image of the plastic cartridge, the electrical interface, the handheld device. (C & D) Realtime GMR signals of BSA and three ovarian cancer biomarkers. (C) Concentrations of IL6, CA125 II, and HE4 are 7.4 pg/mL, 3.7 U/mL and 7.4 pg/mL, respectively. (D) Concentrations of IL6, CA125 II, and HE4 are 200 pg/mL, 100 U/mL and 200 pg/mL, respectively. (A & B) Reprinted with permission from ref. 21. Copyright 2017 American Chemical Society. (C & D) Reprinted with permission from reference [24]. Copyright 2019 Elsevier.
Conclusion
Since biological matrices are nonmagnetic and magnetic labels are stable in different temperature and pH environments. Magnetic sensors have become an emerging research area for biomedical applications. In addition to disease diagnosis, GMR biosensors have also been applied in other areas such as food and drug regulation, genotyping, brain and cardiac imaging, etc. [8]. One issue that confronts the development of GMR biosensing is that most reported literature was testing processed biological samples and requires a long sample preparation time and trained technicians. To overcome this problem, a wash-free bioassay strategy has been proposed by Su D, et al. (2019) [38]. Where the detection antibodies, biological samples, and magnetic labels are premixed to avoid redundant washing steps. This strategy reduced the assay time to a large extent. The other optimization direction for GMR biosensors is employing microfluidic channels with filtration functions in the sensing platform. The integration of such a channel with an on-chip filter will not only allow direct detection of the unprocessed biological samples but also reduce the assay time. Combining wash-free stratifies and microfluidic channels, the next generation of GMR biosensing platform will be ready to be realized. With the development of such a lab-on-chip sensing platform, affordable on-site or at-home detection of cancer biomarkers can be made by patients at ease. Therefore, future GMR biosensors will pave a broad way for daily screening, early-stage diagnosis, and continuous monitoring of cancer.
Acknowledgments
Authors acknowledge funding from Midwest Dairy Food Research Center. This study was also financially Supported by the U.S. Department of Agriculture - National Institute of Food and Agriculture (NIFA) under Award Number 2020-67021-31956.
Conflicts of Interest
Dr. Jian-Ping Wang has equity and royalty interests in Zepto Life Technology LLC, a company involved in the commercialization of GMR Biosensing technology. The University of Minnesota also has equity and royalty interests in Zepto Life Tech LLC. These interests have been reviewed and managed by the University of Minnesota in accordance with its Conflict-of-Interest policies.
References
1. Guo L, Gu X, Zhu X, Sun X. Recent advances in molecular spintronics: Multifunctional spintronic devices. Advanced Materials. 2019 Nov;31(45):1805355. https://doi.org/10.1002/adma.201805355
2. Hirohata A, Takanashi K. Future perspectives for spintronic devices. Journal of Physics D: Applied Physics. 2014 Apr 25;47(19):193001. https://doi.org/10.1088/0022-3727/47/19/193001
3. Makarov A, Windbacher T, Sverdlov V, Selberherr S. CMOS-compatible spintronic devices: a review. Semiconductor Science and Technology. 2016 Oct 14;31(11):113006. https://doi.org/10.1088/0268-1242/31/11/113006
4. Chappert C, Fert A, Van Dau FN. The emergence of spin electronics in data storage. InNanoscience And Technology: A Collection of Reviews from Nature Journals 2010 (pp. 147-157). https://doi.org/10.1142/9789814287005_0015
5. Tsunekawa K, Djayaprawira DD, Nagai M, Maehara H, Yamagata S, Watanabe N, Yuasa S, Suzuki Y, Ando K. Giant tunneling magnetoresistance effect in low-resistance CoFeB? MgO (001)? CoFeB magnetic tunnel junctions for read-head applications. Applied Physics Letters. 2005 Aug 15;87(7):072503. https://doi.org/10.1063/1.2012525
6. Tsang CH, Fontana RE, Lin T, Heim DE, Gurney BA, Williams ML. Design, fabrication, and performance of spin-valve read heads for magnetic recording applications. IBM journal of research and development. 1998 Jan;42(1):103-16. https://doi.org/10.1147/rd.421.0103
7. Stephan AW, Koester SJ. Spin Hall MTJ Devices for Advanced Neuromorphic Functions. IEEE Transactions on Electron Devices. 2020 Jan 13;67(2):487-92. https://doi.org/10.1109/TED.2019.2959732
8. Wu K, Tonini D, Liang S, Saha R, Chugh VK, Wang JP. Giant Magnetoresistance Biosensors in Biomedical Applications. ACS Applied Materials & Interfaces. 2022 Feb 15;14(8):9945-69. https://doi.org/10.1021/acsami.1c20141
9. Chopin C, Torrejon J, Solignac A, Fermon C, Jendritza P, Fries P, Pannetier-Lecoeur M. Magnetoresistive sensor in two-dimension on a 25 μm thick silicon substrate for in vivo neuronal measurements. ACS sensors. 2020 Oct 27;5(11):3493-500. https://doi.org/10.1021/acssensors.0c01578
10. Caruso L, Wunderle T, Lewis CM, Valadeiro J, Trauchessec V, Rosillo JT, Amaral JP, Ni J, Jendritza P, Fermon C, Cardoso S. In vivo magnetic recording of neuronal activity. Neuron. 2017 Sep 13;95(6):1283-91. https://doi.org/10.1016/j.neuron.2017.08.012
11. Fujiwara K, Oogane M, Kanno A, Imada M, Jono J, Terauchi T, Okuno T, Aritomi Y, Morikawa M, Tsuchida M, Nakasato N. Magnetocardiography and magnetoencephalography measurements at room temperature using tunnel magneto-resistance sensors. Applied Physics Express. 2018 Jan 18;11(2):023001. https://doi.org/10.7567/APEX.11.023001
12. Koets M, Van der Wijk T, Van Eemeren JT, Van Amerongen A, Prins MW. Rapid DNA multi-analyte immunoassay on a magneto-resistance biosensor. Biosensors and Bioelectronics. 2009 Mar 15;24(7):1893-8. https://doi.org/10.1016/j.bios.2008.09.023
13. Krishna VD, Wu K, Su D, Cheeran MC, Wang JP, Perez A. Nanotechnology: Review of concepts and potential application of sensing platforms in food safety. Food microbiology. 2018 Oct 1;75:47-54. https://doi.org/10.1016/j.fm.2018.01.025
14. Ren C, Bayin Q, Feng S, Fu Y, Ma X, Guo J. Biomarkers detection with magnetoresistance-based sensors. Biosensors and Bioelectronics. 2020 Oct 1;165:112340. https://doi.org/10.1016/j.bios.2020.112340
15. Nabaei V, Chandrawati R, Heidari H. Magnetic biosensors: Modelling and simulation. Biosensors and Bioelectronics. 2018 Apr 30;103:69-86. https://doi.org/10.1016/j.bios.2017.12.023
16. Baibich MN, Broto JM, Fert A, Van Dau FN, Petroff F, Etienne P, Creuzet G, Friederich A, Chazelas J. Giant magnetoresistance of (001) Fe/(001) Cr magnetic superlattices. Physical review letters. 1988 Nov 21;61(21):2472. https://doi.org/10.1103/PhysRevLett.61.2472
17. Binasch G, Grünberg P, Saurenbach F, Zinn W. Enhanced magnetoresistance in layered magnetic structures with antiferromagnetic interlayer exchange. Physical review B. 1989 Mar 1;39(7):4828. https://doi.org/10.1103/PhysRevB.39.4828
18. Davies JE, Watts JD, Novotny J, Huang D, Eames PG. Magnetoresistive sensor detectivity: A comparative analysis. Applied Physics Letters. 2021 Feb 8;118(6):062401. https://doi.org/10.1063/5.0038187
19. Berkowitz AE, Mitchell JR, Carey MJ, Young AP, Zhang S, Spada FE, Parker FT, Hutten A, Thomas G. Giant magnetoresistance in heterogeneous Cu-Co alloys. Physical Review Letters. 1992 Jun 22;68(25):3745. https://doi.org/10.1103/PhysRevLett.68.3745
20. Xiao G, Wang JQ, Xiong P. Giant magnetoresistance and its evolution in the granular Fe x Ag100− x system (0≤ x≤ 100). Applied physics letters. 1993 Jan 25;62(4):420-2. https://doi.org/10.1063/1.108921
21. Kim D, Marchetti F, Chen Z, Zaric S, Wilson RJ, Hall DA, Gaster RS, Lee JR, Wang J, Osterfeld SJ, Yu H. Nanosensor dosimetry of mouse blood proteins after exposure to ionizing radiation. Scientific reports. 2013 Jul 19;3(1):1-8. https://doi.org/10.1038/srep02234
22. Wang W, Wang Y, Tu L, Klein T, Feng Y, Wang JP. Surface modification for protein and DNA immobilization onto GMR biosensor. IEEE transactions on magnetics. 2012 Dec 21;49(1):296-9. https://doi.org/10.1109/TMAG.2012.2224327
23. Moretti D, DiFrancesco ML, Sharma PP, Dante S, Albisetti E, Monticelli M, Bertacco R, Petti D, Baldelli P, Benfenati F. Biocompatibility of a magnetic tunnel junction sensor array for the detection of neuronal signals in culture. Frontiers in Neuroscience. 2018:909. https://doi.org/10.3389/fnins.2018.00909
24. Wu K, Klein T, Krishna VD, Su D, Perez AM, Wang JP. Portable GMR handheld platform for the detection of influenza a virus. ACS sensors. 2017 Nov 22;2(11):1594-601. https://doi.org/10.1021/acssensors.7b00432
25. Su D, Wu K, Krishna VD, Klein T, Liu J, Feng Y, Perez AM, Cheeran MC, Wang JP. Detection of influenza a virus in swine nasal swab samples with a wash-free magnetic bioassay and a handheld giant magnetoresistance sensing system. Frontiers in microbiology. 2019:1077. https://doi.org/10.3389/fmicb.2019.01077
26. Rizzi G, Lee JR, Dahl C, Guldberg P, Dufva M, Wang SX, Hansen MF. Simultaneous profiling of DNA mutation and methylation by melting analysis using magnetoresistive biosensor array. ACS nano. 2017 Sep 26;11(9):8864-70. https://doi.org/10.1021/acsnano.7b03053
27. Klein T, Wang W, Yu L, Wu K, Boylan KL, Vogel RI, Skubitz AP, Wang JP. Development of a multiplexed giant magnetoresistive biosensor array prototype to quantify ovarian cancer biomarkers. Biosensors and Bioelectronics. 2019 Feb 1;126:301-7. https://doi.org/10.1016/j.bios.2018.10.046
28. Kokkinis G, Cardoso S, Keplinger F, Giouroudi I. Microfluidic platform with integrated GMR sensors for quantification of cancer cells. Sensors and Actuators B: Chemical. 2017 Mar 31;241:438-45. https://doi.org/10.1016/j.snb.2016.09.189
29. Dias TM, Cardoso FA, Martins SA, Martins VC, Cardoso S, Gaspar JF, Monteiro G, Freitas PP. Implementing a strategy for on-chip detection of cell-free DNA fragments using GMR sensors: A translational application in cancer diagnostics using ALU elements. Analytical Methods. 2016;8(1):119-28. https://doi.org/10.1039/c5ay01587a
30. Gao Y, Huo W, Zhang L, Lian J, Tao W, Song C, Tang J, Shi S, Gao Y. Multiplex measurement of twelve tumor markers using a GMR multi-biomarker immunoassay biosensor. Biosensors and Bioelectronics. 2019 Jan 1;123:204-10. https://doi.org/10.1016/j.bios.2018.08.060
31. Su D, Wu K, Saha R, Peng C, Wang JP. Advances in magnetoresistive biosensors. Micromachines. 2019 Dec 26;11(1):34. https://doi.org/10.3390/mi11010034
32. Hall DA, Gaster RS, Makinwa KA, Wang SX, Murmann B. A 256 pixel magnetoresistive biosensor microarray in 0.18 µm CMOS. IEEE journal of solid-state circuits. 2013 Apr 19;48(5):1290-301. https://doi.org/10.1109/JSSC.2013.2245058
33. Xu L, Lee JR, Hao S, Ling XB, Brooks JD, Wang SX, Gambhir SS. Improved detection of prostate cancer using a magneto-nanosensor assay for serum circulating autoantibodies. PloS one. 2019 Aug 12;14(8):e0221051. https://doi.org/10.1371/journal.pone.0221051
34. Nesvet JC, Antilla KA, Pancirer DS, Lozano AX, Preiss JS, Ma W, Fu A, Park SM, Gambhir SS, Fan AC, Neal JW. Giant Magnetoresistive Nanosensor Analysis of Circulating Tumor DNA Epidermal Growth Factor Receptor Mutations for Diagnosis and Therapy Response Monitoring. Clinical Chemistry. 2021 Mar;67(3):534-42. https://doi.org/10.1093/clinchem/hvaa307
35. Ng E, Yao C, Shultz TO, Ross-Howe S, Wang SX. Magneto-nanosensor smartphone platform for the detection of HIV and leukocytosis at point-of-care. Nanomedicine: Nanotechnology, Biology and Medicine. 2019 Feb 1;16:10-9. https://doi.org/10.1016/j.nano.2018.11.007
36. Isiksacan Z, Guler MT, Aydogdu B, Bilican I, Elbuken C. Rapid fabrication of microfluidic PDMS devices from reusable PDMS molds using laser ablation. Journal of Micromechanics and Microengineering. 2016 Feb 3;26(3):035008. https://doi.org/10.1088/0960-1317/26/3/035008
37. Choudhury S, Dutta S, Chatterjee S. Cost-effective template development for the microfluidic device. Micro & Nano Letters. 2019 Aug 8;14(8):860-4. https://doi.org/10.1049/mnl.2018.5411
38. Su D, Wu K, Krishna VD, Klein T, Liu J, Feng Y, Perez AM, Cheeran MC, Wang JP. Detection of influenza a virus in swine nasal swab samples with a wash-free magnetic bioassay and a handheld giant magnetoresistance sensing system. Frontiers in microbiology. 2019:1077. https://doi.org/10.3389/fmicb.2019.01077
