Oral Mucositis during Hematopoietic Stem Cell Transplantation: Novel Findings and Research Directions
Authors: Eniko Gebri1, Attila Kiss2, Tibor Hortobagyi3,4,5*
1Department of Dentoalveolar Surgery, Dental Outpatient Care, Faculty of Dentistry, University of Debrecen, Debrecen, Hungary
2Department of Hematopoietic Transplantation Centre, Faculty of Medicine, University of Debrecen, Debrecen, Hungary
3Institute of Pathology, Albert Szent-Gy
Citation: Gebri E, Kiss A, Hortobágyi T (2021) Oral Mucositis during Hematopoietic Stem Cell Transplantation: Novel Findings and Research Directions, 21st Century Pathol, Volume 1 (2): 108
Abstract
Mucosal barrier injury (MBI), oral (OM), and enteral mucositis (EM), are common and severe toxic complications of high dose conditioning regimen during hematopoietic stem cell transplantation (HSCT). Oral mucositis can cause, beyond the severe decline of quality of life, dissemination of oral microbes leading to fatal sepsis during neutropenia. Also, the associated prolonged hospitalization and frequent nosocomial infections impose severe strains on the health care system. Oral mucositis has several patients- and treatment-related risk factors, with female sex and altered IgA N-glycosylation pattern recently discovered in the context of HSCT. A new aspect is the altered glycosylation pattern of IgA in HSCT, influencing its anti-inflammatory potential. OM treatment is still supportive and palliative despite extensive research. OM has no validated biomarker, although there are promising candidates, such as salivary osteopontin level.
Keywords:
Glycoanalitics; Immunoglobulin A; Oral mucositis; Osteopontin; Progesterone; Saliva
Hematopoietic Stem Cell Transplantation (HSCT)
The number of hematopoietic stem cell transplantations increases every year [1]. The main indications for autologous HSCT are lymphoid malignancies (90%) with plasma cell disorders, comprising 55% of all autologous HSCT patients.
In HSCT the patient?s own haematogenesises and immune system have destroyed the use of appropriate conditioning and immunosuppressive therapy (intensive cytostatic treatment and/or radiotherapy), then healthy mononuclear cells, including CD34+ multipotent hematopoietic stem cells, are administered, which are able to reorganize the myeloid-and lymphopoietic systems [2]. In terms of oral surgery and dentistry, prevention of the acute exacerbation of chronic dental inflammations during cytopenia through appropriate removal, reducing the risks of the development and severity of oral mucositis (OM), and timely recognition of secondary oral cancers and oral graft-versus-host disease (GVHD) during post transplantation care are especially important [3].
Oral mucositis (OM), is a Mucosal Barrier Injury during HSCT
Varying degrees of oral mucositis occurs in 60-100 % of patients during HSCT [4]. The development of mucosal barrier injury is a result of a complex and dynamic biological process involving several molecular and cellular events and affecting all layers of the mucosa (?panmucosal?) [4]. Knowledge of these is especially important in the mapping of new prevention and therapeutic alternatives.
The exact classification of OM enables optimal follow-up of the patient's general status and the assessment of the success of the transplantation. At the same time, it is one of the basic pillars of efficient research [5]. Several scales are known nowadays, of which the classifications of the World Health Organization (WHO) and Oral Assessment Guide (OAG) are most common in clinical practice [5].
Oral mucositis is a disease of multifactorial etiopathogenic origin with several patients (low neutrophil granulocyte count, female sex, poor oral hygiene, etc.) and treatment (TBI, high dose cytostatic therapy) related risk factors (Figure 1) [6].
Of several prevention and treatment alternatives, human recombinant keratinocyte growth factor (hrKGF) is the only prevention alternative approved by the Food and Drug Administration (FDA). However, its routine administration is limited by side effects and high costs [7].
Prediction of ulcerative OM is especially important regarding the efficient and individual plan of oncotherapy. In view of this, therapy modification due to toxicity, dose reduction, or potential hospitalization time can be reduced, significantly improving therapeutic needs.
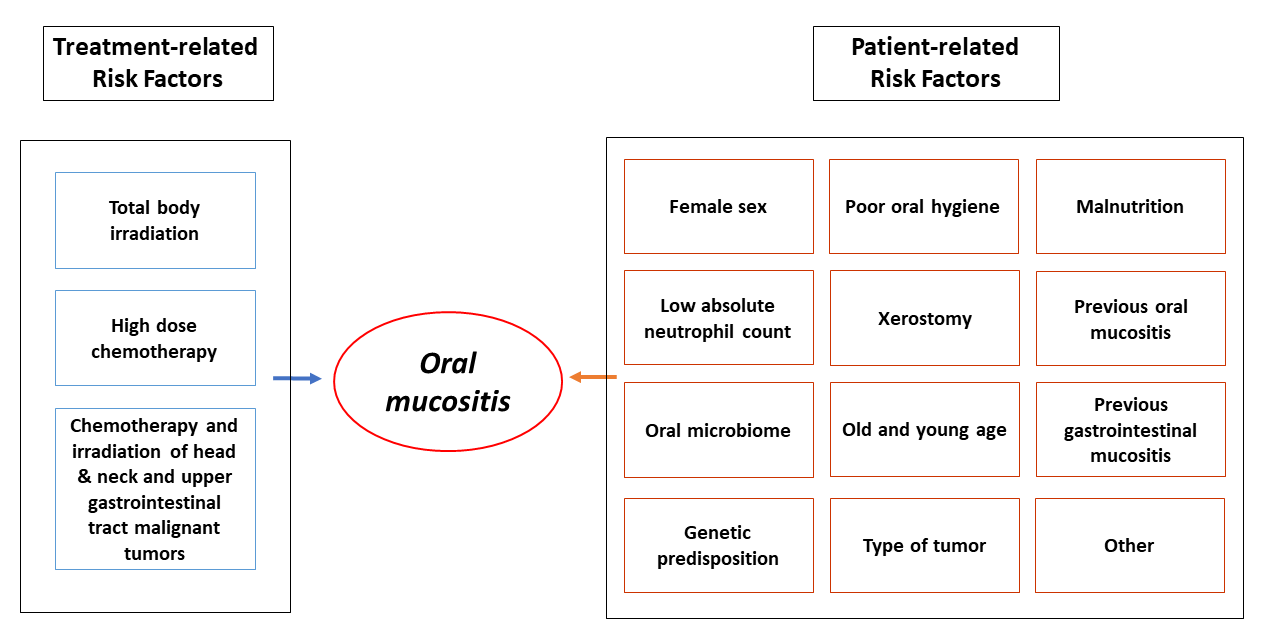
Figure 1: Risk factors of oral mucositis.
Basic Pillars of Oral Immunity
The basic pillars of oral immunity are the oral epithelium, leukocytes, saliva, and the periodontium [8]. The oral epithelium and its associated lamina propria provide a physical barrier that protects the underlying tissues. The action of the immune system?s soldiers (macrophages, dendritic cells, natural killer cells, and polymorphonuclear cells) makes the protective mucosal barrier stronger by producing inflammatory mediators, cytokines, and chemokines. Several defensive contents and functions of saliva and gingival crevicular fluid are essential to the complex and optimal function of oral immunity. Oral mucosal immunity neutralizes the agents which damage the oral cavity, limit the colonization of pathogenic microorganisms, and provide the maintenance of commensal homeostasis. Dysregulation of oral mucosal immunity may result in the development of oral immunopathogenic reactions, common infections, acute and chronic inflammations, and possibly plays an auxiliary role in the development of oral cancer if persists [8].
Effects of Sex Hormones on the Physiology of Oral Cavity
Sex hormones play a pivotal role in the maintenance of homeostasis of the oral cavity and in its regulation. As a consequence of the sex hormone receptors? tissue-specific localization hormones affects the whole oral milieu directly and indirectly. Effects of sex hormones on the oral epithelium, periodontium, microbiome, consumption of saliva, and the function of the immune system can be demonstrated [9]. Estrogen (E2) is primarily an immunostimulant. It regulates the growth, differentiation, and proliferation of lymphocytes, polymorphonuclear (PMN) chemotaxis, antigen presentation, production of cytokines and antibodies, and cell survival. It enhances bloodstream and capillary permeability stimulates the proliferation and keratinization of epithelial cells. Estrogen regulates the production of the extracellular matrix and enhances the proliferation of gingival fibroblasts. It affects wound healing and plays a role in the localization of the dissemination of the dentoalveolar infection by modulating the production of IL-1 [9,10]. On the other hand, progesterone (P4) and androgens are immunosuppressants [10]. P4 stimulates the production of inflammatory mediators. It enhances vascular permeability in the gingival structures. Besides its elevated levels, a decrease in keratinized cells can be observed. Keratinization and karyopycnotic index decrease significantly as a consequence of daily regular administration of synthetic progestins. P4 inhibits the proliferation of gingival fibroblasts. It suppresses the mucosal immune response, inhibiting IgA-associated immune response. Antibacterial activity of neutrophil granulocytes is decreased as a result of the administration of high dose progesterone [9,10].
Role of Secretory Immunoglobulin A (sIgA) and Serum IgA in Mucosal Protection. Importance of Immunoglobulins? Glycosylation
Alterations in N-linked carbohydrate structures of glycoproteins can serve as indicators for several key biochemical mechanisms and offers new paths for biomarker research [11]. Besides IgG, IgA is one of the most abundant glycoproteins in serum and saliva [12]. Glycosylation is essential for the functions of immunoglobulins.
Serum IgA as an anti-inflammatory antibody plays the role of ?silent housekeeper? in regulating infective-inflammatory processes [12]. It has been shown to prevent activation of the complement system and to inhibit phagocytosis, chemotaxis, and antibody-dependent cellular cytotoxicity [12].
The role of the secretory immunoglobulin A (sIgA) is more complex. Salivary sIgA is crucial in immune exclusion via direct interaction with microbial antigens and eliminates viruses by non-virulent immune complex formation. It also neutralizes bacterial lipopolysaccharide (LPS), and maintains commensal homeostasis, thereby preventing disseminating pathogens [13].
As a result of chemotherapy, there is decreased sIgA secretion. During APSCT, serum IgA also decreases [14]. While serum IgA usually returns to the normal level within six or seven months, salivary sIgA level needs up to five years to recover [14].
Osteopontin (OPN)
OPN is a multifunctional, chemokine-like, sialic-acid-rich phosphoglycoprotein, plays a pivotal role in tumor development, progression, inflammation, and mucosal protection impacts on cell survival, proliferation, and invasion [15]. OPN is secreted in body fluids including blood, cerebrospinal fluid, and saliva. Overexpression of OPN in several cancers such as breast cancer, malignant haematological diseases (acute leukemia, lymphoma, multiple myeloma), or oral squamous cell carcinoma (OSCC) [15] predicts poor overall survival, suggesting its role as a prognostic biomarker [15]. OPN is an effective regulator of hematopoietic stem cell homeostasis and neutrophil migration [15]. Its role in mucosal defense, especially against viral pathogens and in tissue destruction with the subsequent repair process, is also essential [1]. Osteopontin may be required for their interaction and co-operative effects in promoting the transition from innate to adaptive responses and the initiation of repair [16].
In view of the above, in our previous studies our aim was to assess risk factors, identify biomarkers, examine the effect of autologous peripheral stem cell transplantation (APSCT) on local immunity and identify new research directions [17-20]. We analyzed the data of 192 patients with the malignant hematological disease who had undergone APSCT. Furthermore, we examined 10 APSCT patients and sampled serum and saliva at four stages of transplantation (day -3/-7; 0; +7; +14) [17-20]. The studies were conducted in accordance with the Declaration of Helsinki, and the protocols were approved by the Regional Institutional Research Ethics Committee, Clinical Centre, University of Debrecen (Ethical license: DE RKEB/IKEB 4948-2018), and Regional and Institutional Committee of Science and Research Ethics (Ethical license: 5570-1/2018/EKU). A written informed consent was obtained from all subjects [17-20].
Discussion
Our retrospective analysis has revealed that female sex is an independent prognostic factor in the development of oral mucositis in lymphoma. That?s why we examined the changes of the two main female sex hormones (estrogen, progesterone) in serum and saliva during transplantation and assessed its correlation with the development of OM. We concluded that elevated progesterone levels at day +7 may play a role in the weakening of the mucosal barriers not only in pre- but also in postmenopause. Our results indicate that monitoring serum progesterone levels in women undergoing APSCT may be a suitable tool in the assessment of mucosal immunity, function, and risk of severe OM [19].
In the next section of the study, we examined the N-glycosylation alteration of serum and salivary immunoglobulin A. To do this, first, a special IgA binding protein had to be designed and produced. Then we confirmed that the developed Z (IgA1) affibody and the high-resolution capillary electrophoresis with laser-induced fluorescent detection (CE-LIF) based glycoanalytical methods provided an efficient and sensitive workflow to detect and monitor IgA glycosylation alterations in serum and saliva [17]. We determined that N-glycosylation alteration of serum and salivary immunoglobulin A is a possible biomarker in oral mucositis during APSCT [18].
The role of osteopontin in mucosal immunity and preservation of epithelial barrier integrity is essential [16]. In our study, we confirmed the importance of osteopontin in mucosal defence during APSCT, too. We concluded that salivary osteopontin could serve as a potential biomarker for oral mucositis and could be a suitable and efficient tool to screen and monitor different endocrine abnormalities. Serum osteopontin has been identified as an efficient marker of malignant haematological diseases during APSCT, too [20].
Conclusion
In our recent studies [17-20], with an in-depth examination of saliva, we identified new etiological factors (elevated P4 and OPN, altered IgA N-glycosylation) which play important role in the development of oral mucositis. These could serve as potential biomarkers and targets of therapeutic interventions (Figure 2). Glycoanalitics has been recommended as an efficient and sensitive method in oral diagnostics and pathology. We have outlined promising new research avenues in relation to oral immunity with relevance to the pathogenesis of oral mucositis.

Figure 2: Potential biomarkers for oral mucositis.
Note: IgA: immunoglobulin A; OPN: osteopontin; P4: progesterone
Financial disclosure
The author declares no relevant financial or non-financial relationships to disclose.
Funding sources
No funding source is to be declared.
Acknowledgment
We are grateful to Professor. Csaba Heged?s,
References
1. Passweg JR, Baldomero H, Chabannon C, et al. (2021) Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transplant 56: 1651-1664. https://doi.org/10.1038/s41409-021-01227-8
2. Ho AD, Haas R, Champlin RE (2000) Hematopoietic stem cell transplantation. Stem Cell Transplantation. CRC Press 604.
3. Bogus?awska-Kapa?a A, Ha?aburda K, Rusyan E, et al. (2017) Oral health of adult patients undergoing hematopoietic cell transplantation. Pre-transplant assessment and care. Ann Hematol 96: 1135-1145. https://doi.org/10.1007/s00277-017-2932-y
4. Sonis ST (2004) The pathobiology of mucositis. Nat Rev Cancer 4: 277-284.https://doi.org/10.1038/nrc1318
5. Quinn B, Stone R, Uhlenhopp M, et al. (2007) Ensuring accurate oral mucositis assessment in the european group for blood and marrow transplantation prospective oral mucositis audit. Eur J Oncol Nurs 11: S10-S18.
https://doi.org/10.1016/S1462-3889(07)70003-0
6. Barasch A, Peterson DE (2003) Risk factors for ulcerative oral mucositis in cancer patients: Unanswered questions. Oral Oncol 39: 91-100. https://doi.org/10.1016/s1368-8375(02)00033-7
7. Jilani S, Kanaan Z, Agarwal R (2014) Palifermin for prevention of oral mucositis in hematological malignancies: present position and future perspectives. Austin J Cancer Clin Res 1: 1-6.
8. Feller L, Altini M, Khammissa RAG, et al. (2013) Oral mucosal immunity. Oral Surg Oral Med Oral Pathol Oral Radiol 116: 576-583. https://doi.org/10.1016/j.oooo.2013.07.013
9. Mariotti A (1994) Sex steroid hormones and cell dynamics in the periodontium. Crit Rev Oral Biol Med 5: 27-53. https://doi.org/10.1177/10454411940050010201
10. Roth JA, Kaeberle ML, Hsu WH (1982) Effect of estradiol and progesterone on lymphocyte and neutrophil functions in steers. Infect Immun 35: 997-1002. https://doi.org/10.1128/iai.35.3.997-1002.1982
11. Ohtsubo K, Marth JD (2006) Glycosylation in cellular mechanisms of health and disease. Cell 126: 855-867. https://doi.org/10.1016/j.cell.2006.08.019
12. Woof JM, Kerr MA (2006) The function of immunoglobulin A in immunity. J Pathol 208: 270-282. https://doi.org/10.1002/path.1877
13. Corthésy B (2013) Multi-faceted functions of secretory IgA at mucosal surfaces. Front Immunol 4: 185. https://doi.org/10.3389/fimmu.2013.00185
14. Norhagen G, Engström PE, Björkstrand B, et al. (1994) Salivary and serum immunoglobulins in recipients of transplanted allogeneic and autologous bone marrow. Bone Marrow Transplant 14: 229-234.
15. Castello LM, Raineri D, Salmi L, et al. (2017) Osteopontin at the crossroads of inflammation and tumor progression. Mediators Inflamm 2017: 4049098. https://doi.org/10.1155/2017/4049098
16. Sodek J, Batista Da Silva AP, Zohar R (2006) Osteopontin and mucosal protection. J Dent Res 85: 404-415. https://doi.org/10.1177/154405910608500503
17. Meszaros B, Kovacs Z, Gebri E, et al. (2020) N-glycomic analysis of Z(IgA1) partitioned serum and salivary immunoglobulin A by capillary electrophoresis. Electrophoresis. Curr Mol Med 20: 781-788. https://doi.org/10.2174/1566524020666200413114151
18. Gebri E, Kovács Z, Mészáros B, et al. (2020) N-glycosylation alteration of serum and salivary immunoglobulin A is a possible biomarker in oral mucositis. J Clin Med 9: 1747. https://doi.org/10.3390/jcm9061747
19. Gebri E, Kiss A, Tóth F, et al. (2020) Female sex as an independent prognostic factor in the development of oral mucositis during autologous peripheral stem cell transplantation. Sci Rep 10: 1-12. https://doi.org/10.1038/s41598-020-72592-5
20. Gebri E, Kiss A, Tóth F, et al. (2021) Salivary osteopontin as a potential biomarker for oral mucositis. Metabolites 11: 208. https://doi.org/10.3390/metabo11040208
