OncoProfiler - A Multi-Cancer Early Detection (MCED) Assay
Authors: Bo Tan1,2,4,5*, Swarna Ganesh2,3,4,5, Rupa Haldavnekar6, Krishnan Venkatakrishnan1,2,3,5
1Keenan Research Center for Biomedical Science, Unity Health Toronto, Canada
2Institute for Biomedical Engineering, Science and Technology (iBEST), Toronto, Canada
3Ultrashort Laser Nanomanufacturing Research Facility, Faculty of Engineering and Architectural Sciences, Toronto Metropolitan University, Canada
4Nano Characterization Laboratory, Faculty of Engineering and Architectural Sciences, Toronto Metropolitan University, Canada.
5Nano-Bio Interface facility, Faculty of Engineering and Architectural Sciences, Toronto Metropolitan University, Canada.
6Department of Science, University of British Columbia, Vancouver, British Columbia, Canada
*Correspondence to: Bo Tan, ENG 170, George Vari Engineering and Computing Centre, Toronto Metropolitan University, 350 Victoria Street, Toronto, Ontario, M5B 2K3, Canada; E-mail: tanbo@ryerson.ca
Received: 08 October 2022; Revised: 03 November 2022; Accepted: 03 November 2022; Published: 05 November 2022
Citation: Tan B, Ganesh S, Haldavnekar R, Venkatakrishnan K (2022) OncoProfiler - A Multi-Cancer Early Detection (MCED) Assay, 21st Century Pathology, Volume 2 (5): 128
Abstract
Laser fabricated SERS nanosensors, OncoProfiler, demonstrated detection sensitivity that sufficient to sense trace amount of tumor-associated content from unprocessed plasma or buffy coat. OncoProfiler enabled the discovery of new biomarkers for cancer detection, which are undetectable with conventional bioassay-based methods. A single test with OncoProfiler could sense multiple biomarkers, which provides high diagnostic accuracy as well as a holistic representation of the spatial and temporal heterogeneity of a tumor. Due to high sensitivity, OncoProfiler has the potential to lead to a non-invasive blood based Multi-Cancer Early Detection (MCED) assay meant for cancer screening and therapeutic monitoring.
Keywords:
Diagnostic Technics and Procedures; Blood; Cancer Early Diagnosis; Raman Scattering
Authors? Contribution:
Conceptualization: BT, KV. Writing ? original draft: SG, BT, RH. Writing ? review & editing: BT, KV.
Conflict of Interest
The authors applied for patents for the device and cancer diagnosis methods.
Introduction
Early cancer detection has been a persistent issue in cancer research for decades. Although liquid biopsy has the potential to solve this ever-persistent issue, conventional liquid biopsy approaches such as DNA microarray, next-gen sequencing, and reverse transcriptase PCR cannot always be reliable due to the low sensitivity, specificity, and extensive sample treatment, usually leading to high false-positives-rate [1], thus skepticism regarding the clinical utility of this technology [2]. In addition, the clinical application of liquid biopsy is hindered by biological barriers such as the lack of cancer-specific biomarkers for asymptomatic cancers, significantly lower biomarkers in circulation at early stages of cancer [3], the short half-life of these biomarkers (up to 3 hours) [4], and the high similarity between healthy and cancer-derived circulating biomaterials [5].
Nanomaterials can aid in developing rapid, cost-effective, and simple substitutes for conventional liquid biopsy approaches since they do not require modification of the analyte using enzymes or multiple amplification steps. Surface-enhanced Raman scattering technology (SERS) has been investigated intensively since it offers essential signal enhancement with high sensitivity, rapid and multiplexing capability, and the ability to provide real-time molecular information without labels [6,7].
Discussion
One of the vital components of SERS is the sensor that amplifies Raman scattering so that ultrahigh sensitivity can be achieved. It is extremely difficult to design and fabricate SERS sensors to function in the biological environment. Most of the SERS-based biosensors made of either noble metal or semiconductors. The former lacks of reasonable reliability and repeatability and the latter provides weak signal [8-11]. It is in this context that we designed a new type of non-metallic SERS nanosensor, OncoProfiler [12-14] using ultrafast laser synthesis. The nanosensor demonstrated limit of detection down to femtomolar concentration with various tumor-associated markers, such as cancer cellular DNA and proteins [8,15]. The chemical stability of the nanosensor and high repeatability [10,16-18] of test make it a clinically applicable tool to diagnosis cancer using patient blood as a primary model.
Figure 1 illustrate the workflow of OncoProfiler. In contrast to existing bioassays, OncoProfiler does not require pre-processing patient blood other than standard blood fractionation. Moreover, comprehensive information of the tumor, including tissue of origin, metastatic states, and prognosis, could be obtained from a single test. Test results could be available within hours of blood withdrawal from patients. Importantly, OncoProfiler is a single piece of equipment, thus, does not require centralized facilities. Taken together, we believe OncoProfile is well suited for low-cost rapid diagnosis of cancer.
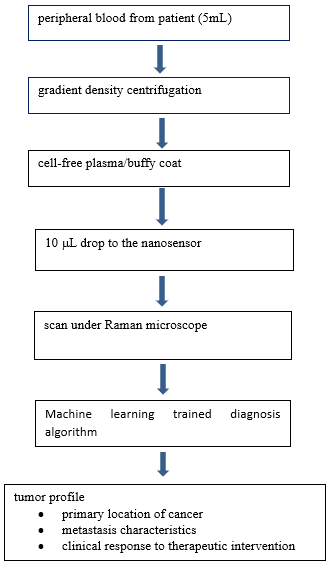
Figure 1: Workflow of OncoProfiler in a clinical setting.
The ultra-sensitivity of Onco Profiler enabled us to discover four tumor associated biomarkers that were hard to detect with any other methods, such as cfDNA, DNA methylation, and extracellular vesicles [12-14,17,19-20]. The pilot validation of OncoProfiler was tested with a small set (22-75) of patient blood samples for 5 different cancer types with diverse tissues of origin. The sensivity and specificity is given in (Table 1). The reported results are based on a single tumor-associated biomarker. If multiple biomarkers are used simultaneously, we can expect a holistic representation of the spatial and temporal heterogeneity of a tumor [16-18] as well as high diagnostic accuracy.
Table 1: Detecting the presence of cancer from blood samples collected from patient with confirmed diagnosis (stage II-IV cancers); blood sample from healthy adult was used as control.
|
Type of cancer |
Specificity |
Sensitivity |
Tumor-associated biomarkers |
Reference |
|
Breast Cancer |
92% |
86% |
ct DNA, NK cell profiling |
[13,17] |
|
Lung Cancer |
96% |
83% |
ctDNA, Exosome profiling |
[13,17,19] |
|
Colorectal Cancer |
92% |
75% |
ctDNA, Exosome Profiling, NK cell profiling |
[13,17,19] |
|
Glioblastoma |
100% |
93.3% |
T cell profiling, Immune exosome profiling |
[12,14,20] |
|
Low Grade Glioma (Astrocytoma and Oligodendroglioma) |
92.15% |
98% |
Immune exosome profiling |
[14] |
Minimally invasive cancer diagnostic methods hold great potential early cancer diagnosis. However, identifying a set of cancer-specific biomarkers with sufficient specificity and sensitivity for early diagnosis of asymptomatic cancers is highly challenging. Although novel biomarkers, such as CSCs, increase the specificity required for early diagnosis, the percentage of biomarkers in a tumor is usually less than 0.1% of the total tumor cells. Hence, only a trace number of tumor-associated biomarkers will be in circulation. The conventional diagnostic methods cannot detect the trace amount of tumor content in circulation since it is beyond their analytical sensitivity. Therefore, existing technologies show only a 10% - 35% sensitivity to detect Stage I breast cancer [21]. OncoProfiler successfully detected the presence of various types of cancer, including hard to detect types, with reasonable sensitivity and specificity from untreated patient blood. It may address the technical challenge of detecting a trace number of tumor-associated biomarkers at the early stage of tumor growth.
Conclusion
OncoProfiler is fabricated using an ultrafast laser-assisted manufacturing technique, which provides the ease of scalability for large-scale mass production. In the future, with the essential preclinical validation studies, the OncoProfiler has the potential to lead to a non-invasive blood test meant for early detection of asymptomatic cancers in a high-risk population and potentially reduce the cost associated with cancer management in the healthcare system.
References
1. Kalogianni DP. Nanotechnology in emerging liquid biopsy applications. Nano convergence. 2021 Dec;8(1):1-23. https://doi.org/10.1186/s40580-021-00263-w
2. Mayer S, Schmidtke-Schrezenmeier G, Buske C, Rücker FG, Barth TF, Möller P, Marienfeld R. Rescue of Non-Informative Circulating Tumor DNA to Monitor the Mutational Landscape in NSCLC. Cancers. 2020 Jul 16;12(7):1917. https://doi.org/10.3390/cancers12071917
3. Alba-Bernal A, Lavado-Valenzuela R, Domínguez-Recio ME, Jiménez-Rodriguez B, Queipo-Ortuño MI, Alba E, Comino-Méndez I. Challenges and achievements of liquid biopsy technologies employed in early breast cancer. EBioMedicine. 2020 Dec 1;62:103100. https://doi.org/10.1016/j.ebiom.2020.103100
4. Bronkhorst AJ, Ungerer V, Holdenrieder S. The emerging role of cell-free DNA as a molecular marker for cancer management. Biomolecular detection and quantification. 2019 Mar 1;17:100087. https://doi.org/10.1016/j.bdq.2019.100087
5. Barbany G, Arthur C, Lieden A, Nordenskjöld M, Rosenquist R, Tesi B, Wallander K, Tham E. Cell?free tumour DNA testing for early detection of cancer–a potential future tool. Journal of Internal Medicine. 2019 Aug;286(2):118-36. https://doi.org/10.1111/joim.12897
6. He F, Wang J, Yin BC, Ye BC. Quantification of exosome based on a copper-mediated signal amplification strategy. Analytical chemistry. 2018 Jun 12;90(13):8072-9. https://doi.org/10.1021/acs.analchem.8b01187
7. Cialla D, Pollok S, Steinbrücker C, Weber K, Popp J. SERS-based detection of biomolecules. Nanophotonics. 2014 Dec 1;3(6):383-411. https://doi.org/10.1515/nanoph-2013-0024
8. Ganesh S, Venkatakrishnan K, Tan B. Quantum scale organic semiconductors for SERS detection of DNA methylation and gene expression. Nature communications. 2020 Feb 28;11(1):1-5. https://doi.org/10.1038/s41467-020-14774-3
9. Haldavnekar R, Venkatakrishnan K, Tan B. Next generation SERS-atomic scale platform for molecular level detection. Applied Materials Today. 2020 Mar 1;18:100529. https://doi.org/10.1016/j.apmt.2019.100529
10. Dharmalingam P, Venkatakrishnan K, Tan B. An atomic-defect enhanced Raman scattering (DERS) quantum probe for molecular level detection–Breaking the SERS barrier. Applied Materials Today. 2019 Sep 1;16:28-41. https://doi.org/10.1016/j.apmt.2019.04.016
11. Powell JA, Venkatakrishnan K, Tan B. Toward universal SERS detection of disease signaling bioanalytes using 3D self-assembled nonplasmonic near-quantum-scale silicon probe. ACS applied materials & interfaces. 2017 Nov 22;9(46):40127-42. https://doi.org/10.1021/acsami.7b15393
12. Dhinakaran AK, Ganesh S, Haldavnekar R, Tan B, Das S, Venkatakrishnan K. Holistic Analysis of Glioblastoma Stem Cell DNA Using Nanoengineered Plasmonic Metasensor for Glioblastoma Diagnosis. Small Methods. 2022 Sep 1:2200547. https://doi.org/10.1002/smtd.202200547
13. Ishwar D, Haldavnekar R, Venkatakrishnan K, Tan B. Minimally invasive detection of cancer using metabolic changes in tumor-associated natural killer cells with Oncoimmune probes. Nature communications. 2022 Aug 4;13(1):1-20. https://doi.org/10.1038/s41467-022-32308-x
14. Ishwar D, Haldavnekar R, Das S, Tan B, Venkatakrishnan K. Glioblastoma Associated Natural Killer Cell EVs Generating Tumour-Specific Signatures: Noninvasive GBM Liquid Biopsy with Self-Functionalized Quantum Probes. ACS nano. 2022 Jul 11;16(7):10859-77. https://doi.org/10.1021/acsnano.2c03055
15. Ganesh S, Venkatakrishnan K, Tan B. Quantum cytosensor for early detection of cancer. Medical Devices & Sensors. 2020 Feb;3(1):e10058. https://doi.org/10.1002/mds3.10058
16. Ganesh S, Venkatakrishnan K, Tan B. Detecting the Origin of Cancer?Mobile Quantum Probe for Single Cancer Stem Cell Detection. Advanced Functional Materials. 2020 Feb;30(9):1907572. https://doi.org/10.1002/adfm.201907572
17. Haldavnekar R, Ganesh S, Venkatakrishnan K, Tan B. Cancer Stem Cell DNA Enabled Real?Time Genotyping with Self?Functionalized Quantum Superstructures—Overcoming the Barriers of Noninvasive cfDNA Cancer Diagnostics. Small Methods. 2022 Apr;6(4):2101467. https://doi.org/10.1002/smtd.202101467
18. Ganesh S, Venkatakrishnan K, Tan B. Early detection and prediction of cancer metastasis–Unravelling metastasis initiating cell as a dynamic marker using self-functionalized nanosensors. Sensors and Actuators B: Chemical. 2022 Jun 15;361:131655. https://doi.org/10.1016/j.snb.2022.131655
19. Haldavnekar R, Venkatakrishnan K, Tan B. Cancer Stem Cell Derived Extracellular Vesicles with Self-Functionalized 3D Nanosensor for Real-Time Cancer Diagnosis: Eliminating the Roadblocks in Liquid Biopsy. ACS nano. 2022 Aug 15;16(8):12226-43. https://doi.org/10.1021/acsnano.2c02971
20. Dhinakaran AK, Dharmalingam P, Ganesh S, Venkatakrishnan K, Das S, Tan B. Molecular Crosstalk between T Cells and Tumor Uncovers GBM-Specific T Cell Signatures in Blood: Noninvasive GBM Diagnosis Using Immunosensors. ACS nano. 2022 Aug 30;16(9):14134-48. https://doi.org/10.1021/acsnano.2c04160
21. Liu MC, Oxnard GR, Klein EA, Swanton CS, Seiden MV, Liu MC, Oxnard GR, Klein EA, Smith D, Richards D, Yeatman TJ. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Annals of Oncology. 2020 Jun 1;31(6):745-59. https://doi.org/10.1016/j.annonc.2020.02.011
