Leucine-rich alpha-2-glycoprotein-1, a Blood and Intracellular Glycoprotein, Interacts with Cytochrome c to Promote Cell Survival and Block Inflammation
Ronald Jemmerson*
Department of Microbiology and Immunology, University of Minnesota, Minneapolis, MN
*Corresponding author: Professor. Ronald Jemmerson, Department of Microbiology and Immunology, 689 23rd Ave. S.E., Minneapolis, MN 55455, USA; E-mail: jemme001@umn.edu
Received: 18 April 2023; Accepted: 29 April 2023; Published: 02 May 2023
Copyright: © 2023 Jemmerson R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Running Title: LRG1 promotes cell survival and inflammation
Citation: Jemmerson R (2023) Leucine-rich Alpha-2-glycoprotein-1, a Blood and Intracellular Glycoprotein, Interacts with Cytochrome c to Promote Cell Survival and Block Inflammation, 21st Century Pathology, Volume 3 (3): 147
Abstract
Leucine-rich alpha-2-glycoprotein-1 (LRG1) is the founding member of the large leucine-rich repeat family of proteins and, like many other members of this family, its function is not completely understood. While attention in the last decade has been given to its role in pathological angiogenesis, LRG1 should be recognized for its role contributing to tissue homeostasis under stress conditions by promoting cell survival and blocking inflammation. Several studies suggest that these functions involve interaction of LRG1 with cytochrome c that translocates from mitochondria into the cytoplasm in initiation of apoptosis and is ultimately released from dying cells. Thus, LRG1 appears to play a regulatory role in pathology, consistent with the observation that mice deficient in LRG1 develop normally and, when healthy, do not display any overt phenotypic abnormality.
Keywords:
LRG1; Cytochrome c; Cell survival; Inflammation; TGF-b1
Introduction
In this era of rapid medical advances and intense investigations into the inner workings of cells, there remain components of the blood whose physiological roles are not completely understood. One such component is leucine-rich alpha-2-glycoprotein-1 (LRG1). LRG1 in the blood is largely produced by the liver and is expressed at lower levels in other tissues (NCBI gene ID 116844). This was the first protein identified that has leucine-rich repeats (LRRs) [1,2]. In humans there are several hundred proteins with LRRs. The repetitive LRR sequence of 20-30 amino acid residues induces a common architecture with a horse-shoe shape where the concave pocket presents a platform for protein interactions (see Figure 1) [3,4]. Indeed, binding of more than one ligand simultaneously has been confirmed for some members of the LRR family [5]. Variation in the components ligated to LRR proteins affect the receptors that are engaged and, consequently, the physiological outcome of the interactions [5].

Figure 1: The computer program Molegro was used to model LRG1. The LRRs are ribbons colored darker blue toward the left side of the molecule. The α-helix near the carboxyl end is colored red. The binding site for Cyt c, a highly positively charged protein, is predicted to lie along the concave surface where the electric field is negative.
Study of LRG1 gene knock-out (lrg1 K.O.) mice provided a clue toward understanding the physiological role of LRG1. These mice have not revealed any developmental abnormality or apparent pathological phenotype under normal conditions [6]. In humans, elevated serum LRG1 has been observed in a variety of disorders including microbial infections, cancer, and tissue-specific disorders such as heart and kidney disease suggesting a role in pathology (reviewed in ref. 6). LRG1 has been shown to play a key role in pathological neovascularization delivering a cell signal in concert with transforming growth factor-β1 (TGF-β1) through the TGF-β1-RII-Alk1-Smad 1/5/8 pathway [7]. Other functions of LRG1 have been identified and likely involve either modifications of this pathway or other pathways through alternative receptors. In addition to the TGF-β1 signaling complex, the receptor latrophilin-2 and the epidermal growth factor receptor have been shown to bind LRG1 [8,9].
A role for extracellular LRG1 in cell survival
Codina R, et al. (2010) were the first researchers to ascribe a function to LRG1 [10]. Previously they showed that LRG1 binds cytochrome c (Cyt c) [11]. Also, it had been reported that Cyt c is released from dying cells [12,13] and extracellular Cyt c can induce apoptosis [14,15]. Codina R, et al. (2010) examined the ability of LRG1 to protect lymphocytes from cell death induced by exogenous Cyt c [10]. Employing cell culture media that had been depleted of LRG1 using Cyt c-coated beads, they showed that LRG1 added to both human and mouse lymphocyte cultures prolonged survival of the cells for two or more days in the presence of exogenous Cyt c. In the absence of additional Cyt c, LRG1 was also effective against the pro-apoptotic effect of Cyt c released from cells within the cultures as the cells began to die over time [10].
The mechanism of LRG1 protection of lymphocytes from Cyt c toxicity was not simply physical blockade. The molecular ratio of LRG1 to Cyt c yielding optimal protection was quite low. Furthermore, mouse lymphocytes were more effectively protected by mouse LRG1 than by human LRG1, although LRG1 from both species was comparable in protecting human lymphocytes [10]. This suggests involvement of a species-specific component in the mouse lymphocytes with which LRG1 interacts, possibly a signal transducer (membrane receptor) in the cells (Figure 2).

Figure 2: Indirect evidence suggests that LRG1 promotes cell survival when bound to extracellular Cyt c that is released from dying cells by signaling through a cell receptor.
Lymphocytes are not the only cells impacted to survive by LRG1. More recently, LRG1 injected at the site of transplanted adipocytes in a mouse fat graft study was shown to promote the survival rate of the cells protecting them from apoptosis [16]. Possible involvement of Cyt c was not examined.
Contradictory effects of LRG1 on cell survival have been observed [17]. For example, in some studies LRG1 was pro-apoptotic and in others it promoted survival. This distinction may be explained by the particular signaling pathway that is activated. Hypothetically, signaling through the TGF-β1-Smad (canonical) pathway could result in apoptosis and signaling through non-canonical pathways could result in cell survival [17]. TGF-β1 was the first LRG1 ligand to be identified [18]. It is produced in a variety of cells and is present in blood. Simultaneous binding of TGF-β1 and Cyt c has been reported [17]. It seems likely that both were bound to LRG1 in the lymphocyte study cited above. The tri-complex of LRG1-TGF-β1-Cyt c may interact with cells through different receptor aggregates than the LRG1-TGF-β1 complex, thus activating signals that promote cell survival instead of apoptosis [17].
A role for intracellular LRG1 in cell survival
LRG1 has 5 glycosylation sites. It appears in the blood as a fully glycosylated protein, whereas LRG1 retained inside cells is only partially glycosylated. This form of LRG1 can enter the cytoplasm where it provides a survival function in a mechanism distinct from that of extracellular LRG1 [19].
A clue to the intracellular survival mechanism was revealed in binding studies. Human LRG1 was shown to bind Cyt c from a variety of species except for yeast Cyt c or mammalian Cyt c produced in yeast [10]. The distinction between a mammalian Cyt c obtained from a natural source and the same protein produced in yeast is a post-translational modification that only occurs in yeast cells, tri-methylation of lysine at position 72 [20]. The same modification was previously shown to inhibit the interaction between Cyt c and its partner in activating apoptosis, Apaf-1 [21]. In a competition experiment, LRG1 did block the binding of Apaf-1 to Cyt c [10] suggesting the possibility that cytoplasmic LRG1 could inhibit the binding between these pro-apoptotic partners in the cytoplasm of cells and block the activation of apoptosis.
To investigate this possibility the level of LRG1 in a breast cancer cell line was altered by gene transfection to increase its expression or gene silencing to decrease its expression [19]. Cells overexpressing LRG1 were found to be more protected from apoptosis induced by hydrogen peroxide than the parental cell line, while cells with a decreased level of LRG1 were more susceptible. Isolation of the cytosols from the cells confirmed the expected relative amounts of LRG1 to be present. Surprisingly, in healthy cells overexpressing LRG1 there was an increase in cytosolic Cyt c which was bound to LRG1 and not Apaf-1 as shown in immunoprecipitation experiments. In apoptotic cells the opposite was true owing to the degradation of LRG1 when the concentration of hydrogen peroxide reached a critical threshold (Figure 3).
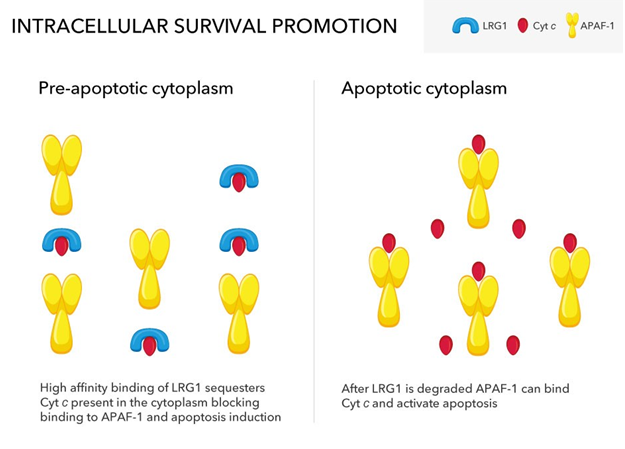
Figure 3: Partially glycosylated LRG1 enters the cytoplasm where it competes with the pro-apoptotic factor Apaf-1 for binding Cyt c that has translocated from mitochondria. LRG1 has a much higher affinity for Cyt c than Apaf-1 allowing LRG1 to effectively sequester Cyt c. Following purposeful apoptotic signaling, LRG1 is degraded, thus allowing Apaf-1 to bind Cyt c.
It was proposed that LRG1 protects cells against apoptosis induction in cells when Cyt c is errantly released from mitochondria in the absence of purposeful pro-apoptotic signaling [19]. As LRG1 expression is increased in a variety of pathologies [5], the intracellular form could protect cells from pro-apoptotic insults emanating from damaged cells in the immediate environment. Other molecules that block the interaction between Cyt c and Apaf-1 have been reported [22]. Perhaps they act either in concert or individually depending upon the particular regulatory mechanisms in play.
Evidence that LRG1 blocks inflammation
Gene-targeted knockout mice are powerful tools to study protein functions in vivo. While lrg1 K.O. mice are normal when healthy, a role for LRG1 emerges in these mice under stress. In a 2016 study of myocardial infarction modelled by ligation of the left coronary artery, fibrosis resulting from chronic inflammation was aggravated in lrg1 K.O. mice compared to control animals [23]. Transplantation of bone marrow cells from normal mice into the lrg1 K.O. mice attenuated fibrosis. These results suggest that LRG1 produced by myeloid cells protects against inflammation in myocardial infarction. Neutrophils, cells in the myeloid lineage, are known to secrete LRG1 and could be the cells implicated in this study [24]. Whether Cyt c plays a role was not examined in this study. However, the myocardial infarction model would result in apoptosis and, likely, the release of Cyt c into the micro-environment making it available to bind LRG1.
More recently, evidence was provided in a mouse obesity study that LRG1 binds Cyt c in inhibiting the production of pro-inflammatory cytokines by macβrophages and blocking the onset of inflammation [25]. It was known that extracellular Cyt c, in addition to inducing apoptosis, can cause arthritis in vivo [26] and engage Toll-like receptor 4 to activate an inflammatory response [27]. In the recent study, adipocytes were shown to secrete LRG1. The amount of LRG1 in serum was increased in mice fed a high fat diet and the increase appeared to be due to production by adipocytes rather than the liver based on lrg1 mRNA levels in the tissues. To examine the effect of LRG1 on inflammation in obesity, LRG1 was overexpressed in genetically obese-prone mice employing a viral vector carrying the lrg1 gene. Compared to lrg1 K.O. mice, overexpression of LRG1 inhibited inflammation as assessed by lack of macrophage accumulation in adipose tissue. In mice over-expressing LRG1, complexes of Cyt c bound to LRG1 were observed in serum by immunoprecipitation and were quantitatively higher than in lean littermates. Furthermore, normal mice fed a high fat diet showed increased circulating levels of LRG1 and Cyt c compared to mice fed a low-fat diet. Finally, bone-marrow derived macrophages were shown to produce pro-inflammatory cytokines when cultured in the presence of Cyt c and the production was decreased in co-cultures of Cyt c and LRG1 [25].
Conclusion
Mice lacking the gene encoding LRG1 develop normally and do not display an overt pathology under normal conditions. However, in disease or stress LRG1 is elevated and promotes cell survival both in the extracellular environment and inside cells. The mechanisms of extracellular and intracellular pro-survival functions are distinct. LRG1 also interferes with the onset of inflammation by blocking production of pro-inflammatory cytokines by macrophages. Evidence from independent studies that these functions involve interaction of LRG1 with Cyt c suggests a role for LRG1 in regulating downstream extracellular events that result from tissue injury.
Contradictory findings in some reports on these functions may be due to involvement of LRG1 ligands other than Cyt c, such as TGF-β1, and different receptors that could modify signaling pathways, thus leading to variation in outcomes.
List of Abbreviations
Cyt c: cytochrome c; K.O: gene-targeted knock-out; LRG1: leucine-rich alpha-2-glycoprotein-1; LRR: leucine-rich repeat; TGF-β1: transforming growth factor-beta1
Conflict of Interest Statement
The author has no competing interests.
References
1. Haupt H, Baudner S. Isolation and characterization of an unknown, leucine-rich 3.1-S-alpha2-glycoprotein from human serum (author's transl). Hoppe-Seyler's Zeitschrift fur physiologische Chemie. 1977 Jun 1;358(6):639-46. PMID: 69600
2. Takahashi N, Takahashi Y, Putnam FW. Periodicity of leucine and tandem repetition of a 24-amino acid segment in the primary structure of leucine-rich alpha 2-glycoprotein of human serum. Proceedings of the National Academy of Sciences. 1985 Apr;82(7):1906-10. https://doi.org/10.1073/pnas.82.7.1906
3. Kobe B, Deisenhofer J. A structural basis of the interactions between leucine-rich repeats and protein ligands. Nature. 1995 Mar 9;374(6518):183-6. http://doi.org/10.1038/374183a0
4. Kobe B, Kajava AV. The leucine-rich repeat as a protein recognition motif. Current opinion in structural biology. 2001 Dec 1;11(6):725-32. https://doi.org/10.1016/s0959-440x(01)00266-4
5. Jackson VA, Mehmood S, Chavent M, Roversi P, Carrasquero M, Del Toro D, Seyit-Bremer G, Ranaivoson FM, Comoletti D, Sansom MS, Robinson CV. Super-complexes of adhesion GPCRs and neural guidance receptors. Nature communications. 2016 Apr 19;7(1):11184. https://doi.org/10.1038/ncomms11184
6. Camilli C, Hoeh AE, De Rossi G, Moss SE, Greenwood J. LRG1: an emerging player in disease pathogenesis. Journal of Biomedical Science. 2022 Dec;29(1):1-29. https://doi.org/10.11886/s12929-022-00790-6
7. Wang X, Abraham S, McKenzie JA, Jeffs N, Swire M, Tripathi VB, Luhmann UF, Lange CA, Zhai Z, Arthur HM, Bainbridge JW. LRG1 promotes angiogenesis by modulating endothelial TGF-β signalling. Nature. 2013 Jul 18;499(7458):306-11. https://doi.org/10.1038/nature12345
8. Yin GN, Kim DK, Kang JI, Im Y, Lee DS, Han AR, Ock J, Choi MJ, Kwon MH, Limanjaya A, Jung SB. Latrophilin-2 is a novel receptor of LRG1 that rescues vascular and neurological abnormalities and restores diabetic erectile function. Experimental & Molecular Medicine. 2022 May;54(5):626-38. https://doi.org/10.1038/s12276-022-00773-5
9. Xie ZB, Zhang YF, Jin C, Mao YS, Fu DL. LRG-1 promotes pancreatic cancer growth and metastasis via modulation of the EGFR/p38 signaling. Journal of Experimental & Clinical Cancer Research. 2019 Dec;38:1-2. https://doi.org/10.1186/s13046-019-1088-0
10. Codina R, Vanasse A, Kelekar A, Vezys V, Jemmerson R. Cytochrome c-induced lymphocyte death from the outside in: inhibition by serum leucine-rich alpha-2-glycoprotein-1. Apoptosis. 2010 Feb;15:139-52. https://doi.org/10.1007/s10495-009-0412-0
11. Cummings C, Walder J, Treeful A, Jemmerson R. Serum leucine-rich alpha-2-glycoprotein-1 binds cytochrome c and inhibits antibody detection of this apoptotic marker in enzyme-linked immunosorbent assay. Apoptosis. 2006 Jul;11:1121-9. https://doi.org/10.1007/s10495-006-8159-3
12. Renz A, Berdel WE, Kreuter M, Belka C, Schulze-Osthoff K, Los M. Rapid extracellular release of cytochrome c is specific for apoptosis and marks cell death in vivo. Blood, The Journal of the American Society of Hematology. 2001 Sep 1;98(5):1542-8. https://doi.org/10.1182/blood.v98.5.1542
13. Jemmerson R, LaPlante B, Treeful A. Release of intact, monomeric cytochrome c from apoptotic and necrotic cells. Cell Death & Differentiation. 2002 May;9(5):538-48. https://doi.org/10.1038/sj.cdd.4400981
14. Ahlemeyer B, Klumpp S, Krieglstein J. Release of cytochrome c into the extracellular space contributes to neuronal apoptosis induced by staurosporine. Brain research. 2002 May 3;934(2):107-16. https://doi.org/10.1016/s0006-8993(02)02365-x
15. Hiraoka Y, Yamada T, Goto M, Das Gupta TK, Chakrabarty AM. Modulation of mammalian cell growth and death by prokaryotic and eukaryotic cytochrome c. Proceedings of the National Academy of Sciences. 2004 Apr 27;101(17):6427-32. https://doi.org/10.1073/pnas.0401631101
16. Ho CK, Zheng D, Sun J, Wen D, Wu S, Yu L, Gao Y, Zhang Y, Li Q. LRG?1 promotes fat graft survival through the RAB31?mediated inhibition of hypoxia?induced apoptosis. Journal of Cellular and Molecular Medicine. 2022 Jun;26(11):3153-68. https://doi.org/10.1111/jcmm.17280
17. Jemmerson R. Paradoxical roles of leucine-rich α2-glycoprotein-1 in cell death and survival modulated by transforming growth factor-beta 1 and cytochrome c. Frontiers in Cell and Developmental Biology. 2021 Oct 8;9:744908. https://doi.org/10.3389/fcell.2021.744908
18. Saito K, Tanaka T, Kanda H, Ebisuno Y, Izawa D, Kawamoto S, Okubo K, Miyasaka M. Gene expression profiling of mucosal addressin cell adhesion molecule-1+ high endothelial venule cells (HEV) and identification of a leucine-rich HEV glycoprotein as a HEV marker. The Journal of Immunology. 2002 Feb 1;168(3):1050-9. https://doi.org/10.4049/jimmunol.168.3.1050
19. Jemmerson R, Staskus K, Higgins L, Conklin K, Kelekar A. Intracellular leucine-rich alpha-2-glycoprotein-1 competes with Apaf-1 for binding cytochrome c in protecting MCF-7 breast cancer cells from apoptosis. Apoptosis. 2021 Feb;26:71-82. https://doi.org/10.1007/s10495-020-01647-9
20. Hickey DR, Jayaraman K, Goodhue CT, Shah J, Fingar SA, Clements JM, Yumi H, Tsunasawa S, Sherman F. Synthesis and expression of genes encoding tuna, pigeon, and horse cytochromes c in the yeast Saccharomyces cerevisiae. Gene. 1991 Aug 30;105(1):73-81. https://doi.org/10.1016/0378-1119(91)90515-d
21. Kluck RM, Ellerby LM, Ellerby HM, Naiem S, Yaffe MP, Margoliash E, Bredesen D, Mauk AG, Sherman F, Newmeyer DD. Determinants of cytochrome c pro-apoptotic activity: The role of lysine 72 trimethylation. Journal of Biological Chemistry. 2000 May 26;275(21):16127-33. https://doi.org/10.1074/jbc.275.21.16127
22. Shakeri R, Kheirollahi A, Davoodi J. Apaf-1: Regulation and function in cell death. Biochimie. 2017 Apr 1;135:111-25. https://doi.org/10.1016/j.biochi.2017.02.001
23. Kumagai S, Nakayama H, Fujimoto M, Honda H, Serada S, Ishibashi-Ueda H, Kasai A, Obana M, Sakata Y, Sawa Y, Fujio Y. Myeloid cell-derived LRG attenuates adverse cardiac remodelling after myocardial infarction. Cardiovascular Research. 2016 Feb 1;109(2):272-82. https://doi.org/10.1093/cvr/cvv273
24. Druhan LJ, Lance A, Li S, Price AE, Emerson JT, Baxter SA, Gerber JM, Avalos BR. Leucine rich α-2 glycoprotein: a novel neutrophil granule protein and modulator of myelopoiesis. PLoS One. 2017 Jan 12;12(1):e0170261. https://doi.org/10.1371/journal.pone.0170261
25. Choi CH, Barr W, Zaman S, Model C, Park A, Koenen M, Lin Z, Szwed SK, Marchildon F, Crane A, Carroll TS. LRG1 is an adipokine that promotes insulin sensitivity and suppresses inflammation. Elife. 2022 Nov 8;11:e81559. https://doi.org/10.7554/eLife.81559
26. Pullerits R, Bokarewa M, Jonsson IM, Verdrengh M, Tarkowski A. Extracellular cytochrome c, a mitochondrial apoptosis-related protein, induces arthritis. Rheumatology. 2005 Jan 1;44(1):32-9. https://doi.org/10.1093/rheumatology/keh406
27. Gouveia A, Bajwa E, Klegeris A. Extracellular cytochrome c as an intercellular signaling molecule regulating microglial functions. Biochimica et Biophysica Acta (BBA)-General Subjects. 2017 Sep 1;1861(9):2274-81. https://doi.org/10.1016/j.bbagen.2017.06.017
