Implications of Polyamine Metabolism for the Pathogenesis of SLE: from Cases of Monogenic Lupus with SAT1 Gene Loss-of-function
Author: Masayuki Mizui*
Department of Nephrology, Osaka University Graduate School of Medicine, Suita, Japan
*Correspondence to: Masayuki Mizui, Department of Nephrology, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita, Osaka 5650871, Japan; Tel: +81668793857 Fax: +81668793230; E-mail: mmizui@kid.med.osaka-u.ac.jp
Received: 14 October 2022; Accepted: 22 October 2022; Published: 31 October 2022
ORCID: https://orcid.org/0000-0003-2543-8108
Conflict of Interest Statement
No potential conflict of interest relevant to this article was reported.
Citation: Mizui M (2022) Implications of Polyamine Metabolism for the Pathogenesis of SLE: from Cases of Monogenic Lupus with SAT1 Gene Loss-of-function, 21st Century Pathology, Volume 2 (5): 130
Abstract
Systemic Lupus Erythematosus (SLE) mediated by a single gene mutation is rare but gives insights into the disease pathogenesis. The present report from Xu et al that SLE is caused by the loss-of-function of spermidine/spermine N1-acetyltransferase (SAT1) gene provides unprecedented evidence that polyamine metabolism, rather than glucose or lipid metabolism, is directly associated with the development of SLE. The functional significance of polyamines in SLE is a contentious issue. In this short commentary, the relevance of polyamine metabolism in the immune system is summarized and how polyamines link to SLE is discussed.
Keywords:
SLE; Polyamines; SAT1; NETs
Introduction
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease characterized by multiple organ inflammation mediated through immune complex of autoantibodies [1]. Disease pathogenesis involves a variety of factors such as gender, environment, and genetic background. In recent years, genome-wide association study (GWAS) has identified various genes susceptible to SLE [2], and genetic risk score, calculated from multiple risk gene combinations, could predict disease onset and renal involvement [3]. However, each risk gene alone is not strong enough to predict disease. In contrast, monogenic or inherited lupus, SLE caused by a single gene mutation, is rare but could be a great help for understanding disease pathogenesis by the facilitation of reverse translational research. Major genetic variants in monogenic lupus are those related to autoantigen processing and inflammatory response regulation, such as DNA degrading enzymes, apoptosis, and interferon signaling [4]. The present study is novel as well as important in that the loss-of-function mutation of the X-chromosome-related gene spermidine/spermine N1-acertyltransferase (SAT1) causes childhood-onset SLE, which has not been ever reported as a risk gene before [5]. While SAT1 is a polyamine metabolism-regulating gene, the importance of polyamine in immune cell function has been proven recently [6,7]. Here, the implication of polyamine metabolism in the immune system is briefly summarized, and how this genetic abnormality is involved in disease pathogenesis is discussed.
Biological function of polyamines in immune cells
Polyamines are aliphatic polycations present in all cells and play an important role in mediating biological functions necessary for cellular growth and survival [8]. Putrescine (Put), Spermidine (Spd) and Spermine (Spm) are naturally occurring polyamines, and the arginine-ornithine-Put synthesis pathway is the rate-limiting step. Polyamine production is primarily regulated by ornithine decarboxylase (ODC), and ODC transcription is controlled by c-Myc, a critical regulator of cellular growth and metabolic reprogramming [9]. The importance of polyamine biosynthesis is clear from the fact that ODC-deficient mice are embryonic lethal and the growth of heterozygous mice is severely impaired [10]. Polyamines have diverse functions, including the regulation of ion channels, maintenance of chromatin structure, stabilization of DNA replication, transcription, translation, and they are also closely involved in cell death processes such as apoptosis and ferroptosis. Moreover, polyamines are known to function as a free radical scavenger, protecting cells from oxidative stress [11].
The first report on the role of polyamines in the immune system was in 1977, it was shown that both innate and acquired immunity were inhibited by exogenous polyamine administration [12]. Subsequent studies have revealed a variety of physiological activities beyond immunosuppression. Recently, polyamine metabolism is reported to be important for effector T-cell differentiation and function. Wagner et al reported that polyamine metabolism was enhanced in pathogenic Th17 cells, and deletion or pharmacological inhibition of genes for polyamine synthesis alleviated autoimmune diseases [6]. Puleston DJ, et al. (2021) demonstrated that normal T cell differentiation was perturbated when enzymes for polyamine synthesis were depleted, and inflammatory T cells were increased [7]. Although these two reports share the same point of view that polyamines are important for normal effector T cell differentiation, they have different observations when polyamine synthesis is inhibited: one shows that the Th17/Treg balance is disrupted and Tregs become significant, and the other shows that Th17 is decreased but IFNG production tends to increase. Conversely, Carriche et al demonstrated that Spd administration suppressed Th17 differentiation and enhanced Treg differentiation [13]. These discrepancies indicate that polyamines may exert different effects dependent on cellular conditions.
Polyamines have been reported to act not only on lymphocytes but also on monocytes/macrophages and dendritic cells. Addition of spermine to human monocytes/macrophages inhibits the induction of inflammatory cytokines such as TNFA, IL-1 and IL-6 in response to LPS stimulation. In dendritic cells, Spd induces the expression of indoleamine 2,3-dioxygenase 1 (IDO1), which is involved in suppressive dendritic cell function [8]. Thus, the actions of polyamines in myeloid cells are mainly explained by their immunosuppressive mechanisms. Interestingly, a recent report demonstrates that when macrophages engulf apoptotic cells, exogenously imported polyamines, but not polyamines from retention of apoptotic cell metabolites, play a role for suppressing the induction of inflammatory responses [14].
SAT1 deficiency and lupus
SAT1, located on the X chromosome, is a catabolic enzyme in polyamine metabolism. SAT1 is a polyamine acetylase, together with polyamine oxidation by spermine oxidase (SMOX), is responsible for the polyamine catabolic pathway. N1-acetylated polyamines by SAT1 are either excreted from the cells or returned to polyamines if they are oxidized. A variety of transcriptional regulatory systems for SAT1 have been reported, including Nrf-2, NFkB, p53, and interferon signaling [11]. Increased polyamine catabolism such as pharmacological induction of SAT1 has anti-tumor effect through polyamine depletion, resulting in reduced cell growth and survival [15]. SAT1 overexpression has also been found to induce mitochondrial alterations, apoptosis and ferroptosis. By contrast, deletion of either SAT1 or SMOX alone was shown to result in a mild phenotype. SAT1-deficient mice have been reported to develop insulin resistance at 12 months of age, with no effect on development or growth, even though organ polyamine levels are increased [16]. On the other hand, deletion of both SAT1 and SMOX has been shown to cause severe neurological symptoms [17].
In the current report, the authors identified two novel hemizygous SAT1 mutations in two families of male patients with childhood-onset hereditary SLE: The p.Asp40Tyr mutation is a spliced donor site mutation that results in splicing defects. The other, p.Glu92Leufs*6, is likely to cause mRNA decay. Both mutations are loss-of-function mutations. They also generated SAT1 p.Glu92Leufs*6 knock-in mice and found that 10-week-old knock-in male mice potentially exhibit SLE-like clinical findings and lupus nephritis. These mice had reduced neutrophil counts, potentially predisposing them to NETosis with impaired autophagy function. In addition, they found that the number of regulatory T cells was also decreased. However, the SLE-like lesions in these mice were mild and not progressive [5].
Discussion
Controversies of polyamines in autoimmune diseases
As described above, biological functions of polyamines in the regulation of CD4+Th cell differentiation remain controversial. Treatment with ODC inhibitor reduces experimental autoimmune encephalomyelitis (EAE) by a decrease in pathogenic IL-17 positive T cells and an increase in Tregs [6]. In contrast, adoptive transfer of T cells lacking ODC into RAG1 mice resulted in exacerbation of autoimmune enteritis, accompanied by an increase in IFNg-positive inflammatory T cells [7]. These inconsistent results may be related to a combination of factors, including temporal and quantitative issues in which cells require endogenous polyamines, such as during differentiation and proliferation, and the ability to take up exogenous polyamines.
The first report on SLE was that administration of a polyamine synthesis inhibitor suppressed T cell proliferation and reduced lupus lesions in MRL/lpr mice, a mouse model of SLE, although the mechanism has not been elucidated [18]. This seems to be contrary to the theory of current report. Furthermore, it was reported that polyamine levels of Spm, SpD, N1-acetylspermidine, and N1-acetylcadaverin are significantly lower in human SLE blood than in healthy controls [19], while sera from SLE patients in the present paper showed that Put and N1-acetylspermidine were clearly elevated. However, data from both of these studies are concentrations of polyamines in blood and do not reflect tissue amount or intracellular polyamines.
Possible links between polyamines and SLE
Stabilization of NETs by polyamines
Neutrophil extracellular traps (NETs) are aggregates containing nucleoproteins and other proteins released from neutrophils, which induce cell death (NETosis). Dysregulation of NETosis has also been implicated in autoimmune diseases, including SLE [20]. Brooks hypothesized that increased polyamines induce the formation of nuclear aggregates of polyamines (NAPs), which stabilize nucleoproteins, and can also activate peptidylarginine deiminase 4 (PAD4), an enzyme important in NETosis, leading to NETosis that releases NAPs (NAPs in NETs), thereby contributing to prolonged autoantigen exposure [21]. This hypothesis has been partially proven to be correct by the report that chlorinated polyamines forms NET-conjugate by cross-linking of NET-specific proteins, which leads to the stability of NET and is essential for its biological function [22].
SAT1 p.Glu92Leufs*6 knock-in mice exhibit a potentially NETosis-prone neutrophil trait accompanied with reduced number of peripheral neutrophils. They found that SAT1 is strongly expressed on neutrophils [5]. The increase in intracellular polyamines due to inactivation of SAT1 may contribute to enhanced autoantigen exposure and activation of NETosis signals.
Polyamine-induced Z-DNA formation as an autoantigenic trait
In mammalian cells, polyamines are 90% bound to RNA and DNA. One effect of polyamines on DNA structure is a transition from the normal right-handed double helix structure (B-DNA) to left-handed (Z-DNA) Z-DNA. Z-DNA can be recognized by Z-DNA binding protein 1 (ZBP1) in the cytoplasm and could induce inflammatory responses [23]. Z-DNA/Z-RNA, is recognized and edited for silencing by adenosine deaminase acting on RNA type I (ADAR1). In particular, ADAR1 dysfunction or gene deficiency induces Z-RNA-mediated interferon responses that leads to the severe autoimmune disease, Aicardi-Goutieres syndrome [24]. Increased Spm stabilizes Z-DNA along with NAP, and could be immunogenic. Anti-Z-DNA antibodies have been found to occur in human SLE and mouse models of SLE, suggesting that Z-DNA is a structure that can induce an autoimmune-responses.
Conclusion
Polyamines are essential for cell activity and survival, and disturbances in their homeostasis may contribute to the development of SLE. Possible mechanisms by which perturbation of polyamine metabolism leads to autoimmunity are summarized in figure. Although the importance of polyamines in immune cells has been demonstrated, further investigation is needed, as inhibition of polyamine synthesis can either exacerbate or alleviate lymphocytic inflammation. Increased NETosis by SAT1-deficiency is described, however, it is likely but has not yet been directly illustrated that stabilization of NETs and Z-DNA by polyamine could induce autoimmunity. In any case, the fact that polyamines are linked to SLE is a definite milestone toward elucidating the mechanism of disease pathogenesis.
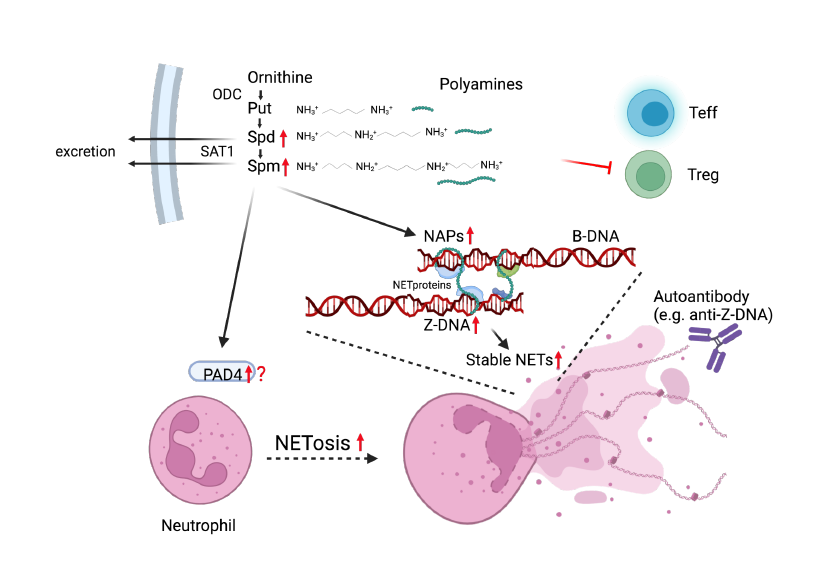
Figure 1: Proposed links between SAT1 deficiency and autoimmunity increased intracellular polyamines through SAT1 loss-of-function increase Z-DNA, NAPs and could activate PAD4 leading to NETosis. Stable
Figure 1: Proposed links between SAT1 deficiency and autoimmunity increased intracellular polyamines through SAT1 loss-of-function increase Z-DNA, NAPs and could activate PAD4 leading to NETosis. Stable NETs by NAPs tends to induce autoimmune responses. Red arrows and inhibitor are possible alterations with SAT1 deficiency.
NAP: nuclear aggregates of polyamine, PAD4: peptidyl arginine deiminase 4, NET: neutrophil extracellular trap.
References
1. Tsokos GC. Autoimmunity and organ damage in systemic lupus erythematosus. Nature immunology. 2020 Jun;21(6):605-14. https://doi.org/10.1038/s41590-020-0677-6
2. Ortíz-Fernández L, Martín J, Alarcón-Riquelme ME. A Summary on the Genetics of Systemic Lupus Erythematosus, Rheumatoid Arthritis, Systemic Sclerosis, and Sjögren’s Syndrome. Clinical Reviews in Allergy & Immunology. 2022 Jun 24:1-20. https://doi.org/10.1007/s12016-022-08951-z
3. Chen L, Wang YF, Liu L, Bielowka A, Ahmed R, Zhang H, Tombleson P, Roberts AL, Odhams CA, Cunninghame Graham DS, Zhang X. Genome-wide assessment of genetic risk for systemic lupus erythematosus and disease severity. Human molecular genetics. 2020 May 15;29(10):1745-56. https://doi.org/10.1093/hmg/ddaa030
4. Lo MS. Monogenic lupus. Current rheumatology reports. 2016 Dec;18(12):1-7. https://doi.org/10.1007/s11926-016-0621-9
5. Xu L, Zhao J, Sun Q, Xu X, Wang L, Liu T, Wu Y, Zhu J, Geng L, Deng Y, Awgulewitsch A. Loss-of-function variants in SAT1 cause X-linked childhood-onset systemic lupus erythematosus. Annals of the Rheumatic Diseases. 2022 Aug 17. https://doi.org/10.1136/ard-2022-222795
6. Wagner A, Wang C, Fessler J, DeTomaso D, Avila-Pacheco J, Kaminski J, Zaghouani S, Christian E, Thakore P, Schellhaass B, Akama-Garren E. Metabolic modeling of single Th17 cells reveals regulators of autoimmunity. Cell. 2021 Aug 5;184(16):4168-85. https://doi.org/10.1016/j.cell.2021.05.045
7. Puleston DJ, Baixauli F, Sanin DE, Edwards-Hicks J, Villa M, Kabat AM, Kami?ski MM, Stanckzak M, Weiss HJ, Grzes KM, Piletic K. Polyamine metabolism is a central determinant of helper T cell lineage fidelity. Cell. 2021 Aug 5;184(16):4186-202. https://doi.org/10.1016/j.cell.2021.06.007
8. Chia TY, Zolp A, Miska J. Polyamine Immunometabolism: Central Regulators of Inflammation, Cancer and Autoimmunity. Cells. 2022 Mar 5;11(5):896. https://doi.org/10.3390/cells11050896
9. Bello-Fernandez C, Packham G, Cleveland JL. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proceedings of the National Academy of Sciences. 1993 Aug 15;90(16):7804-8. https://doi.org/10.1073/pnas.90.16.7804
10. Meehan TF, Conte N, West DB, Jacobsen JO, Mason J, Warren J, Chen CK, Tudose I, Relac M, Matthews P, Karp N. Disease model discovery from 3,328 gene knockouts by The International Mouse Phenotyping Consortium. Nature genetics. 2017 Aug;49(8):1231-8. https://doi.org/10.1038/ng.3901
11. Zahedi K, Barone S, Soleimani M. Polyamines and Their Metabolism: From the Maintenance of Physiological Homeostasis to the Mediation of Disease. Medical Sciences. 2022 Jul 15;10(3):38. https://doi.org/10.3390/medsci10030038
12. Byrd WJ, Jacobs DM, Amoss MS. Synthetic polyamines added to cultures containing bovine sera reversibly inhibit in vitro parameters of immunity. Nature. 1977 Jun;267(5612):621-3. https://doi.org/10.1038/267621a0
13. Carriche GM, Almeida L, Stüve P, Velasquez L, Dhillon-LaBrooy A, Roy U, Lindenberg M, Strowig T, Plaza-Sirvent C, Schmitz I, Lochner M. Regulating T-cell differentiation through the polyamine spermidine. Journal of Allergy and Clinical Immunology. 2021 Jan 1;147(1):335-48. e311. https://doi.org/10.1016/j.jaci.2020.04.037
14. McCubbrey AL, McManus SA, McClendon JD, Thomas SM, Chatwin HB, Reisz JA, D’Alessandro A, Mould KJ, Bratton DL, Henson PM, Janssen WJ. Polyamine import and accumulation causes immunomodulation in macrophages engulfing apoptotic cells. Cell reports. 2022 Jan 11;38(2):110222. https://doi.org/10.1016/j.celrep.2021.110222
15. Stewart TM, Dunston TT, Woster PM, Casero RA. Polyamine catabolism and oxidative damage. Journal of Biological Chemistry. 2018 Nov 30;293(48):18736-45. https://doi.org/10.1074/jbc.TM118.003337
16. Niiranen K, Keinänen TA, Pirinen E, Heikkinen S, Tusa M, Fatrai S, Suppola S, Pietilä M, Uimari A, Laakso M, Alhonen L. Mice with targeted disruption of spermidine/spermine N1?acetyltransferase gene maintain nearly normal tissue polyamine homeostasis but show signs of insulin resistance upon aging. Journal of cellular and molecular medicine. 2006 Oct;10(4):815-27. https://doi.org/10.2755/jcmm010.004.02
17. Zahedi K, Brooks M, Barone S, Rahmati N, Murray Stewart T, Dunworth M, Destefano-Shields C, Dasgupta N, Davidson S, Lindquist DM, Fuller CE. Ablation of polyamine catabolic enzymes provokes Purkinje cell damage, neuroinflammation, and severe ataxia. Journal of neuroinflammation. 2020 Dec;17(1):1-21. https://doi.org/10.1186/s12974-020-01955-6
18. Gunnia UB, Amenta PS, Seibold JR, Thomas TJ. Successful treatment of lupus nephritis in MRL-lpr/lpr mice by inhibiting ornithine decarboxylase. Kidney international. 1991 May 1;39(5):882-90. https://doi.org/10.1038/ki.1991.111
19. Kim HA, Lee HS, Shin TH, Jung JY, Baek WY, Park HJ, Lee G, Paik MJ, Suh CH. Polyamine patterns in plasma of patients with systemic lupus erythematosus and fever. Lupus. 2018 May;27(6):930-8. https://doi.org/10.1177/0961203317751860
20. Yu Y, Su K. Neutrophil extracellular traps and systemic lupus erythematosus. Journal of clinical & cellular immunology. 2013 Apr 1;4. https://doi.org/10.4172/2155-9899.1000139
21. Brooks WH. Increased polyamines alter chromatin and stabilize autoantigens in autoimmune diseases. Frontiers in immunology. 2013 Apr 17;4:91. https://doi.org/10.3389/fimmu.2013.00091
22. Csomós K, Kristóf E, Jakob B, Csomós I, Kovács G, Rotem O, Hodrea J, Bagoly Z, Muszbek L, Balajthy Z, Cs?sz É. Protein cross-linking by chlorinated polyamines and transglutamylation stabilizes neutrophil extracellular traps. Cell death & disease. 2016 Aug;7(8):e2332. https://doi.org/10.1038/cddis.2016.200
23. Jiao H, Wachsmuth L, Kumari S, Schwarzer R, Lin J, Eren RO, Fisher A, Lane R, Young GR, Kassiotis G, Kaiser WJ. Z-nucleic-acid sensing triggers ZBP1-dependent necroptosis and inflammation. Nature. 2020 Apr;580(7803):391-5. https://doi.org/10.1038/s41586-020-2129-8
24. Jiao H, Wachsmuth L, Wolf S, Lohmann J, Nagata M, Kaya GG, Oikonomou N, Kondylis V, Rogg M, Diebold M, Tröder SE. ADAR1 averts fatal type I interferon induction by ZBP1. Nature. 2022 Jul;607(7920):776-83. https://doi.org/10.1038/s41586-022-04878-9
