Cytochrome c Keeps on Giving—A Conformational Change Detected in Situ with an Antibody Probe Adds to the Intricacies of Its Cellular Functions
Professor. Ronald Jemmerson*
Department of Microbiology and Immunology, University of Minnesota, Minneapolis, MN
*Corresponding author: Professor Emeritus. Ronald Jemmerson, Department of Microbiology and Immunology, 689 23rd Ave. S.E., Minneapolis MN 55455, USA; E-mail: jemme001@umn.edu
Received: 29 January 2024; Accepted: 19 February 2024; Published: 22 February 2024
Copyright: © 2024 Jemmerson R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Jemmerson R (2024) Cytochrome c Keeps on Giving—A Conformational Change Detected in Situ with an Antibody Probe Adds to the Intricacies of Its Cellular Functions. 21st Century Pathology, Volume 4 (1): 157
Abstract
Beyond its well-established roles in electron transport within mitochondria and activation of the apoptotic peptidase cascade in the cytoplasm, cytochrome c (Cyt c) has been implicated in several functions at other locations, both inside and outside the cell. A monoclonal antibody (mAb) that detects a conformational change in Cyt c in situ has recently been recovered from “the ashes” by genetic engineering and should be useful in furthering analysis of the roles of Cyt c in the cell, in particular, in the nucleus. The approach employed to define the conformational specificity of anti-Cyt c mAbs could be applied to discover antibodies specific for other conformationally-altered antigens that may be useful probes in studies of cell biology.
Keywords:
Cytochrome c; Conformational alteration; Monoclonal antibody; Nuclear function
Running title: mAb probe for conformationally-altered Cyt c
Introduction
Margoliash E and Schejter A were prescient in 1996 when they wrote “Cytochrome c has become an extremely popular protein, and its popularity has no sign of stabilizing, but rather of continuing to increase [1].” Even if they were aware of the report that cytochrome c (Cyt c) plays a role in activating the apoptotic peptidase (caspase) cascade which appeared late in 1996 [2], additional unexpected roles for Cyt c beyond electron transport and apoptosis activation have since been identified [3].
The finding that Cyt c plays a critical role in apoptosis activation was originally met with skepticism when first submitted for publication [2]. Further experimentation involving depletion of Cyt c from fractionated cellular extracts employing a mAb to immunoprecipitate Cyt c provided convincing evidence that Cyt c, more specifically native Cyt c, and not a contaminant in the cell extract was responsible for apoptosis activation. In addition, evidence that Cyt c is released from mitochondria into the cytosol in apoptosis was provided using a mAb reactive with denatured Cyt c that, unlike the mAb specific for native Cyt c, was useful in Western blot experiments [2]. Now, a mAb that detects a conformationally-altered form of Cyt c in cells, resurrected from “the ashes” by genetic engineering, promises to further our understanding of Cyt c functions, in particular, in the nucleus [4-6].
Multiple Binding Partners in Several Cellular Locations Implicate a Variety of Functions for Cyt c
Discovery that a mitochondrial component, well known to be involved in bioenergetics and the life of a cell, also played a role signaling the initiation of cell death in the cytoplasm was quite illuminating. It raised the possibility that other mitochondrial components could have roles in regulating cell processes and that Cyt c itself could have additional functions outside mitochondria [7]. Examples of some of the known or putative functions and binding partners for Cyt c in various cellular locations are listed in Table 1. This topic has been reviewed more extensively elsewhere [3].
Table 1: Examples of Cyt c binding partners and functions in various cellular locations.
| Cellular Location | Binding Partner | Function | Reference |
|---|---|---|---|
| Mitochondrial inter membrane space | Cyt c reductase, oxidase | Electron transport | [8] |
| Mitochondrial outer membrane | Cardiolipin | Peroxidase activity, facilitation of Cyt c translocation to the cytoplasm | [9] |
| Endoplasmic reticulum | Inositol (1,4,5) trisphosphate receptors | Amplifies Cyt c translocation from mitochondria to the cytoplasm | [10] |
| Zymogen and growth hormone granules | unknown | Secretion of Cyt c for extracellular functions (?) | [11] |
| Cytoplasm | Apaf-1 | Activation of apoptosis | [2] |
| 14-3-3ε | Cyt c binds 14-3-3ε and blocks inhibition of Apaf-1 | [12] | |
| Hsp 27 | Blocks Cyt c binding to Apaf-1 and inhibits apoptosis activation | [13] | |
| LRG1 | [14] | ||
| Nucleus | Chromatin-binding factors | Chromatin reorganization in DNA repair | [3,15] |
| Extracellular | LRG1 TLR4 |
Lymphocyte survival, inhibition of inflammation Activation of brain microglia |
[16,17] [18] |
The classical function of Cyt c, as a resident in the space between the inner and outer mitochondrial membrane, is to transfer electrons from cytochrome reductase to cytochrome oxidase in the respiratory chain [8]. In this location a fraction of the Cyt c molecules is electrostatically bound to cardiolipin. In the early stage of apoptosis, the tail of cardiolipin inserts into the hydrophobic core of Cyt c altering its conformation and rendering it functional as a peroxidase. Then, as a cardiolipin oxygenase, the altered Cyt c facilitates its own translocation through the outer mitochondrial membrane into the cytoplasm [9]. This process is amplified by the release of calcium from the endoplasmic reticulum as a consequence of Cyt c interacting with inositol 1,4,5-trisphosphate receptors at that site early following its translocation from mitochondria [10].
The interaction of Cyt c with apoptotic peptidase activating factor 1 (Apaf-1) in the cytoplasm, resulting in the activation of the caspase cascade, is now well established [2]. This process is regulated by a number of factors, some of which bind Cyt c [12-14]. The factor 14-3-3? acts to inhibit Apaf-1 [12]. When 14-3-3? is blocked by binding Cyt c, caspase activation is heightened. Two other cytosolic factors, heat shock protein 27 (Hsp27) and leucine-rich alpha-2-glycoprotein-1 (LRG1), compete with Apaf-1 for binding Cyt c, thus blocking activation of the caspase cascade [13,14].
During apoptosis Cyt c has been observed in the nucleus by immunofluorescence and cell fractionation [15]. When Cyt c was added to isolated nuclei, acetylated histone 2A was released. Chromatin condensation also occurred in isolated nuclei in response to Cyt c when combined with crude cytosol implicating unknown cytosolic factors as well [15]. More recently, Cyt c has been shown to bind histone chaperones and appears to interfere with their binding to histones, ultimately affecting the reorganization of chromatin. It has been proposed that this facilitates the DNA repair process [3].
Cyt c has been observed in secretory granules in the rat pancreas and anterior pituitary under normal conditions employing immunogold labeling in electron microscopy [11]. This suggests that Cyt c may play an extracellular role under normal conditions. Extracellular Cyt c induces cultured human and mouse lymphocytes to undergo apoptosis by an unknown mechanism and this can be inhibited by serum LRG1 [16]. Serum LRG1 can also block the pro-inflammatory effect of Cyt c [17]. Furthermore, extracellular Cyt c has been shown to activate brain microglial cells involved in immune defense by engaging toll-like receptor 4 (TLR4) [18]. Taken together these findings indicate that Cyt c is a damage-associated molecular pattern (DAMP) that appears to serve as a harbinger of cell damage in the extracellular environment [19]. By interacting with certain other molecules Cyt c may minimize the spread of damage to neighboring cells.
A well-studied conformational change in Cyt c is detected in situ with a mAb probe
It is well established that in alkaline pH or when bound to phospholipids Cyt c undergoes a conformational change indicated by loss of the 695 nm band in the absorption spectrum. This results from the displacement of methionine 80 from its ligation to the heme iron, the so-called sixth axial ligand, that is concomitant with a limited destabilization of the polypeptide backbone. In Fig. 1 this ligation to the heme iron is on the left side of the heme, colored pink, with the iron atom colored red.
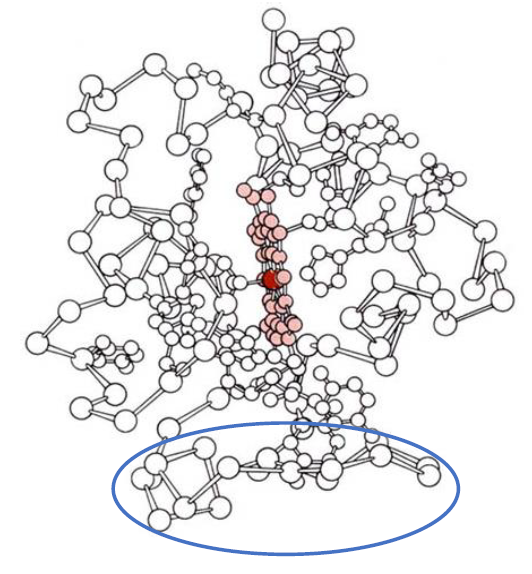
Figure 1: The polypeptide backbone of Cyt c is shown with the covalently-attached heme colored pink and its central iron atom colored red. The side chains of histidine at position 18 and methionine at position 80, which are ligated to the heme iron, are also shown. The omega loop consisting of residues 40-54 is indicated by the blue ellipse.
We identified a mAb (mAb 1D3) reactive with phospholipid-bound Cyt c that binds in the region of Residues 40-54, an omega loop (Fig. 1, blue ellipse [4]). The epitope for mAb 1D3 is not exposed in native Cyt c but, as occurs when Cyt c is bound to phospholipid, is exposed in some molecules when native Cyt c is non-covalently adsorbed to a microtiter plate in enzyme-linked immunosorbent assay (ELISA). In a competitive ELISA, mAb 1D3 bound longer peptides of Cyt c but not the native protein. As shown in Fig. 2, with intact Cyt c attached to the assay plate, the polypeptide fragment containing residues 1-80, derived by cyanogen bromide cleavage, in solution was more effective inhibiting the binding of the mAb to the assay plate than was the 40-54 peptide, which in turn was more effective than the shorter, 41-48 peptide. A fragment containing residues 1-65 was slightly less effective than the 1-80 fragment and native Cyt c did not inhibit the binding of the mAb to assay plate-bound Cyt c (not shown). These results indicate that mAb 1D3 recognizes an epitope on conformationally-altered Cyt c that is native-like but not strictly native [20].
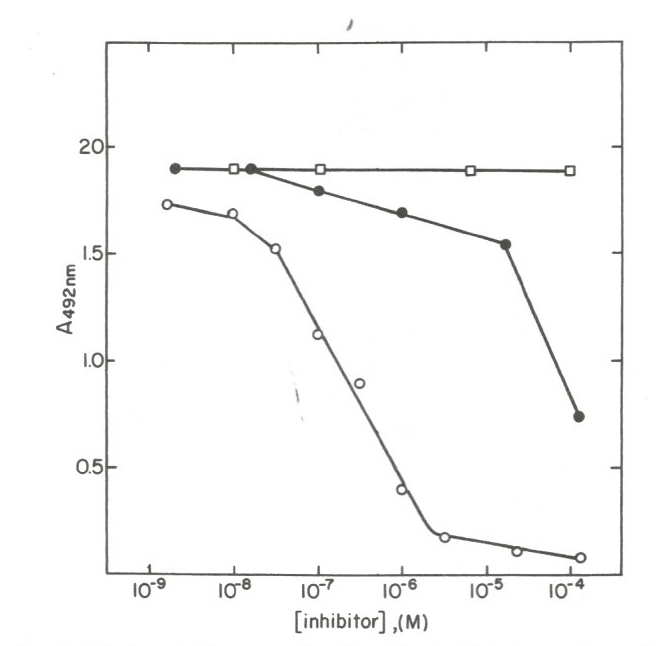
Figure 2: Results of a competitive ELISA are shown in which several peptides were examined for their ability to inhibit the binding of mAb 1D3 to assay plate-bound Cyt c. The epitope for the mAb is localized to the 40-54 omega loop. Higher affinity binding of the mAb to the longer 1-80 polypeptide (open circles) than to the 40-54 peptide (closed circles), weaker binding to the 41-48 peptide (closed circles), and failure to bind native Cyt c in solution (not shown) indicate that the conformationally-altered form of Cyt c recognized by the mAb is native-like but not strictly native [20]. (Note that in other experiments weaker inhibition by the 41-48 was observed, although in the experiment shown no inhibition was apparent). Note: Reused with permission from reference [20]. Copyright 1995 CRC press.
By immunofluorescence this mAb detected Cyt c in mitochondria of a T hybridoma cell line early in apoptosis, even earlier than the appearance of other apoptotic markers [4]. In retrospect this could be interpreted as the mAb detecting Cyt c bound to cardiolipin in the early phase leading to the translocation of Cyt c into the cytoplasm. The mAb 1D3 has since proven useful to detect a conformationally-altered form of Cyt c in the nucleus [5]. Mutation of methionine 80 to alanine in HeLa cells, which results in loss of the sixth axial ligand to the heme, led to the variant Cyt c translocating to the cytoplasm and nucleus in the absence of apoptosis activation. In the same study, peroxynitrite treatment of wild-type cells resulted in nuclear translocation of Cyt c. Peroxynitrite causes chemical nitration of tyrosines and, in Cyt c, disruption of the Met80-heme iron ligation [5].
The original hybridoma secreting mAb 1D3 was lost due to a leak in the liquid nitrogen tank in which the cells were stored. Recently, the mAb was resurrected by molecular cloning from the amino acid sequences of its heavy and light chains [6]. The engineered mAb was shown to have the same binding properties as the original mAb and to detect conformationally-altered Cyt c in the nucleus of both peroxynitrite- and hydrogen peroxide-treated cells, conditions that also induce DNA damage [6].
The recombinant mAb 1D3 and mAbs specific for native Cyt c can now be used to determine whether the conformational alteration recognized by mAb 1D3 is required for Cyt c translocation to the nucleus, which form is active in the nucleus, and under what conditions the variant arises in cells, among other questions.
A general approach to identify mAbs specific for conformationally-altered antigens
Application of mAbs to probe conformationally-altered antigens is an avenue for discovery that remains largely untraveled. There is a simple approach to identify such mAbs without knowledge of the molecular basis for the conformational change or even whether a conformational variant exists.
Typically, to elicit an antibody response in an animal, an antigen is emulsified in mineral oil along with an adjuvant to enhance the immune response. The emulsification causes denaturation of some antigen molecules [21]. Consequently, mAbs specific for native and conformationally-altered forms of the antigen arise in immunized animals. After immortalizing the antibody-producing cells by fusion with a myeloma cell, culture fluid from the cloned hybridomas is screened for mAbs that bind the antigen. Employing a solid-phase assay where antigen is attached non-covalently to a solid support, again some molecules denature upon adherence as noted above for Cyt c. Competition with the native antigen in solution can be employed to distinguish mAbs reactive with native versus conformationally-altered variants as mAbs specific for the variants will bind denatured antigen on the solid-support even in the presence of excess native antigen [21].
Once the conformational specificities of the mAbs have been defined, the mAbs can be assessed for detecting antigen in cells, for example, under normal, differentiative, stressed, and apoptotic conditions. Caution must be taken to choose an appropriate fixation procedure prior to cell labeling as this step can lead to denaturation of antigens.
Like mAb 1D3, the mAb employed in Ref. 2 to identify Cyt c in a Western blot to demonstrate the presence of Cyt c in the cytosol (mAb 7H8) was identified in this manner and subsequently defined as recognizing a short peptide at the carboxyl terminus (Residues 93-104). Both mAbs 1D3 and 7H8 were tested in normal and apoptotic cells and only mAb 1D3 was found to be useful for cell labeling in those experiments [4].
Conclusion
The discovery that Cyt c plays dual roles in mitochondria and the cytoplasm was illuminating. It extended the function of mitochondria beyond bioenergetics to signaling in the regulation of the cell and opened the door to the discovery of Cyt c functions beyond electron transport and apoptosis activation. Multiple roles for Cyt c both inside and outside the cell have been identified, including its role as a DAMP (Fig. 3). Clues toward understanding the nuclear function of Cyt c, such as a role in chromatin reorganization and DNA repair, have been reported. The re-engineered mAb 1D3 should be useful in furthering studies of the nuclear function of Cyt c.
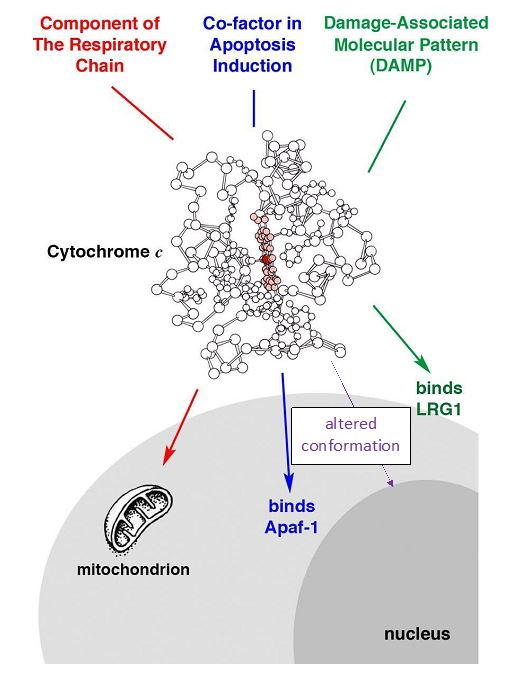
Figure 3: Under certain conditions Cyt c is found at various sites within the cell and in the extracellular space. It has not been determined whether the conformational alteration recognized by mAb 1D3 is the unique requirement for Cyt c translocation to the nucleus or whether native Cyt c can also translocate there.
Application of mAb probes specific for conformationally-altered forms of antigens to the study of cell biology is a frontier largely unexplored. The approach employed to identify the conformational specificity of anti-Cyt c mAbs 1D3 and 7H8 could be applied to other antigens in the discovery of novel mAb probes that may be useful uncovering conformational alterations affecting function.
Abbreviations
Apaf-1: apoptotic peptidase activating factor-1; Cyt c: cytochrome c; DAMP: damage-associated molecular pattern; ELISA: enzyme-linked immunosorbent assay; Hsp27: heat shock protein 27; LRG1: leucine-rich alpha-2-glycoprotein-1; mAb: monoclonal antibody; TLR4: toll-like receptor 4
Conflict of Interest Statement
The University of Minnesota holds licenses for the Cyt c-specific mAbs employed in Ref. 2.
References
1. Margoliash E, Schejter A (1996) How does a small protein become so popular? a succinct account of the development of our understanding of cytochrome c. Cytochrome c: A Multidisciplinary Approach. 3-31.
2. Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996 Jul 12;86(1):147-57. https://doi.org/10.1016/S0092-8674(00)80085-9
3. González?Arzola K, Velázquez?Cruz A, Guerra?Castellano A, Casado?Combreras MÁ, Pérez?Mejías G, Díaz?Quintana A, et al. New moonlighting functions of mitochondrial cytochrome c in the cytoplasm and nucleus. FEBS letters. 2019 Nov;593(22):3101-19. https://doi.org/10.1002/1873-3468.13655
4. Jemmerson R, Liu J, Hausauer D, Lam K-P, Mondino A, Nelson RD. A conformational change in cytochrome c of apoptotic and necrotic cells is detected by monoclonal antibody binding and mimicked by association of the native antigen with synthetic phospholipid vesicles. Biochemistry. 1999 Mar 23;38(12):3599-3609. https://doi.org/10.1021/bi9809268
5. Godoy LC, Muñoz-Pinedo C, Castro L, Cardaci S, Schonhoff CM, King M, et al. Disruption of the M80-Fe ligation stimulates the translocation of cytochrome c to the cytoplasm and nucleus in nonapoptotic cells. Proc Natl Acad Sci U S A. 2009 Feb 24;106(8):2653-8. https://doi.org/10.1073/pnas.0809279106
6. Tomasina F, Martínez J, Zeida A, Chiribao ML, Demicheli V, Correa A, Quijano C, Castro L, Carnahan RH, Vinson P, Goff M. De novo sequencing and construction of a unique antibody for the recognition of alternative conformations of cytochrome c in cells. Proce Natl Acad Sci U S A. 2022 Nov 22;119(47):e2213432119. https://doi.org/10.1073/pnas.2213432119
7. Chandel NS. Evolution of mitochondria as signaling organelles. Cell Metabol. 2015 Aug 4;22(2):204-6. https://doi.org/10.1016/jcmet.2-15.05.013
8. Slater EC. Keilin, cytochrome, and the respiratory chain. J Biol Chem. 2003 May 9;278(19):16455-61. https://doi.org/10.1074/jbc.X200011200
9. Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005 Sep 14; 1(4):223-232. https://doi.org/10.1038/nchembio727
10. Boehning D, Patterson RL, Sedaghat Ll, Glebova NO, Kurosaki T, Snyder SH. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003 Nov 9;5(12):1051-1061. https://doi.org/10.1038/ncb1063
11. Soltys BJ, Andrews DW, Jemmerson R, Gupta RS. Cytochrome-c localizes in secretory granules in pancreas and anterior pituitary. Cell Biol Int. 2001 Aug 8; 25(4):331-338 https://doi.org/10.1006/cbir.2000.0651
12. Elena-Real CA, Diaz-Quintana A, Gonzalez-Arzola K, Velazquez-Campoy A, Orzaez M, Lopez-Rivas A, Gil-Caballero S, De la Rosa MA, Diaz-Moreno I. Cytochrome c speeds up caspase cascade activation by blocking 14-3-3?-dependent Apaf-1 inhibition. Cell Death Dis. 2018 Mar 6;9(3):365. https://doi.org/10.1038/s41419-018-0408-1
13. Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000 Sep;2(9):645-652. https://doi.org/10.1038/35023595
14. Jemmerson R, Staskus K, Higgins L, Conklin K, Kelekar A. Intracellular leucine-rich alpha-2-glycoprotein-1 competes with Apaf-1 for binding cytochrome c in protecting MCF-7 breast cancer cells from apoptosis. Apoptosis. 2021 Feb;26(1-2):71–82. https://doi.org/10.1007/s10495-020-01647-9
15. Nur-E-Kamal A, Gross SR, Pan Z, Balklava Z, Ma J, Liu LF. Nuclear translocation of cytochrome c during apoptosis. J Biol Chem. 2004 Jun 11;279(24):24911-24914. https://doi.org/10.1074/jbc.C400051200
16. Codina R, Vanasse A, Kelekar A, Vezys V, Jemmerson R. Cytochrome c-induced lymphocyte death from the outside in: inhibition by serum leucine-rich alpha-2-glycoprotein-1. Apoptosis. 2010 Oct 23;15(2):139-152. https://doi.org/10.1007/s10495-009-0412-0
17. Choi CHJ, Barr W, Zaman S, Model C, Park A, Koenen M, et al. LRG1 is an adipokine that promotes insulin sensitivity and suppresses inflammation. Elife. 2022 Nov 8;11e81559. https://doi.org/10.7554/eLife.81559
18. Gouveia A, Bajwa E, Klegeris A. Extracellular cytochrome c as an intercellular signaling molecule regulating microglial functions. Biochim Biophys Acta Gen Subj. 2017 Sep;1861(9):2274-2281. https://doi.org/10.1016/j.bbagen.2017.06.017
19. Eleftheriadis T, Pissas G, Liakopoulos V, Stefanidis J. Cytochrome c as a potentially clinical useful marker of mitochondrial and cellular damage. Front Immunol. 2016 Jul 20; 7:279. https://doi.org/10.3389/fimmu.2016.00279
20. Zegers ND, Boersma WJ, Claassen E. Immunological recognition of peptides in medicine and biology. CRC press; 1995 Jul 19. https://doi.org/10.3109/08820139609059299
21. Jemmerson R. Antigenicity and native structure of globular proteins: Low frequency of peptide reactive antibodies. Proc Natl Acad Sci U S A. 1987 Dec;84:9180-9184. https://doi.org/10.1073/pnas.84.24.9180